Table of Contents
In the quest for technological improvements in the extractive metallurgy of zinc, some efforts have been made to develop a one-step pyrometallurgical process for direct reduction of zinc sulfide to metallic zinc. The earliest endeavors were those of Eulenstein in 1912 and Peterson in 1913 who both attempted to reduce zinc sulfide with iron in an electric furnace. Good extraction of zinc was obtained, but problems with zinc condensation and blue powder (finely divided ZnO) formation appeared to play a large role in discontinuing the investigations. Kelley reported a detailed thermodynamic study of the process in the temperature range 1,300° to 1,800° K, together with the corresponding reaction for reducing PbS. He obtained excellent agreement with the data of Eulenstein. Kelley’s calculations indicated the technical feasibility of conducting the direct reduction at about 1,600° K to produce zinc vapor at atmospheric pressure from a liquid matte phase containing ZnS, FeS, and Fe. Gross and Warrington studied the kinetics of the reaction in the temperature range 900° to 1,000° C. Their experiments were conducted in vacuum and indicated that the reaction would proceed practically to completion in 1 to 3 hours depending on the temperature. Under these conditions the ZnS, Fe, and FeS remained as solids. Bethune and Pidgeon measured the equilibrium vapor pressure of zinc obtained both in iron reduction and in copper reduction of ZnS at 850° to 1,000° C.
The present investigation was undertaken to determine kinetic data in the temperature range 1,000° to 1,400° C where the reaction product FeS would vary from solid to liquid, and to investigate the feasibility of using iron scrap in a semicontinuous direct-reduction process. The production of sulfurous gas and the distribution of metallic impurities between metal (zinc) and FeS matte phases were also determined.
Theoretical Considerations
The theoretical metallurgy of zinc sulfide reduction with iron has been well described.
The solid state reaction and free energy equations from Kelley’s work are shown in equations 1 and 2.
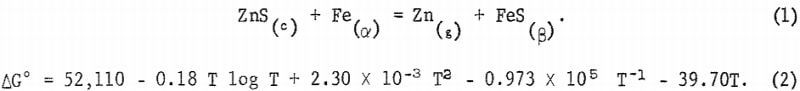
The reaction and free energy equations for FeS and Fe as liquids are shown in equations 3 and 4.
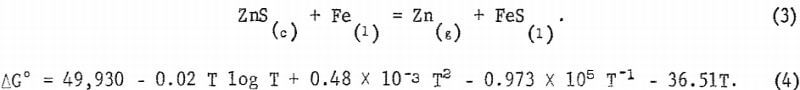
The thermodynamic relationship ΔG = -RT ln K along with equations 2 and 4 enables the calculation at any desired temperature of K = αFeS PZn/αFeαZnS from which the equilibrium zinc pressure can be computed,
G = Gibbs free energy,
R = the gas constant,
T = the temperature in degrees K,
and K = the equilibrium constant.
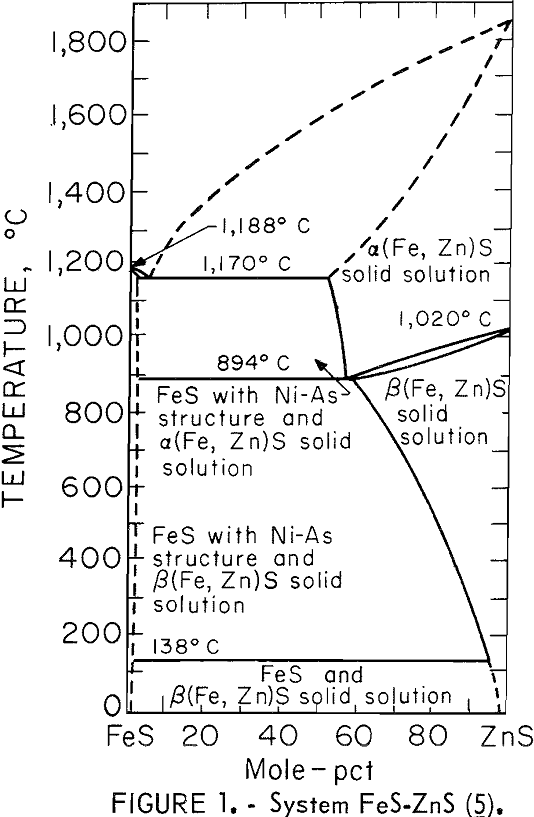
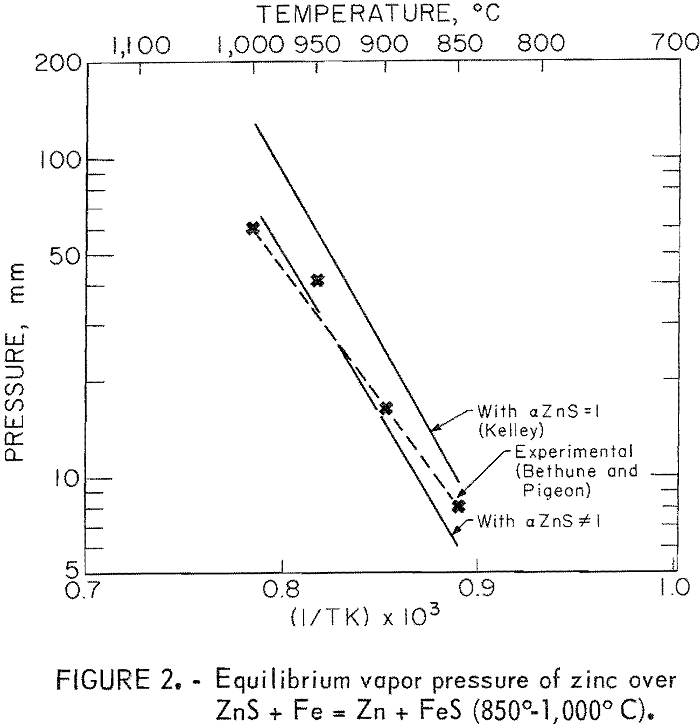
In the temperature range 850° to 1,000° C, both Kelley and Bethune assumed that ZnS was a pure solid and used unit activity for ZnS in their calculations. However, later work by Kullerud has shown the solid solubility of FeS in ZnS to be quite large (fig. 1), thereby making the assumption of unit activity invalid. Using the data of Kullerud and assuming an ideal solution, the equilibrium vapor pressure of zinc has been recalculated and is shown in figure 2 along with the works of Kelley and Bethune. The agreement between the experimental data and the recalculated values is much improved.
At temperatures above the eutectic isotherm, the remaining unreacted ZnS would likely be in solution in the FeS matte and have very low activity (fig. 1). This means that the amount of iron required to satisfy the equilibrium ZnS(c) +Fe(l) = Zn(g) + FeS(l) is increased above that calculated by Kelley.
The reader is referred to Kelley’s original work for a detailed analysis of the problem of PbS and SO2 production.
Materials
Four zinc concentrate samples were provided by the Bunker Hill Co. and the National Zinc Co., Inc. The three National Zinc Co. samples were from the following sources:
Bruce concentrate from Cyprus Mine Corp., Hillside, Ariz,
Camp Bird concentrate from Federal Resources Corp. Ouray, Colo.
Zinc Mine Works concentrate from United States Steel Corp., Jefferson City, Tenn.
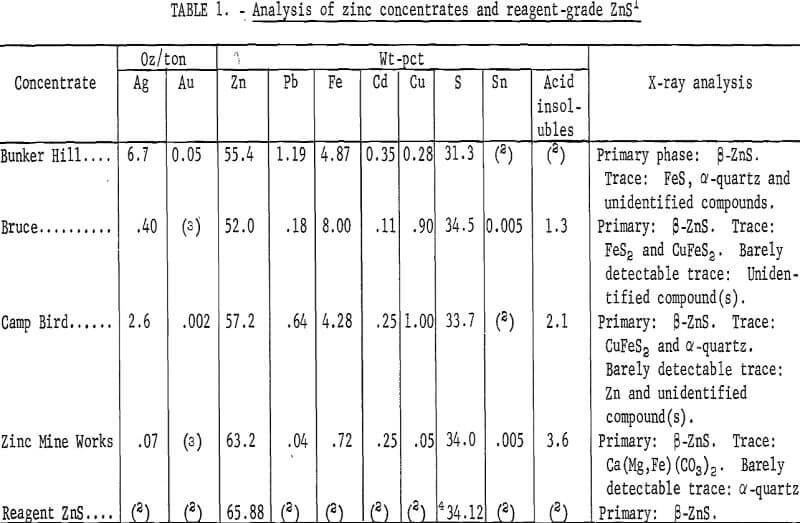
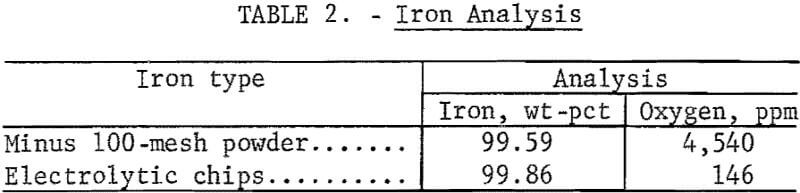
The analyses of the concentrates and of reagent-grade ZnS are shown in table 1. Analyses of iron and oxygen content of high-purity iron reductants purchased from commercial sources are shown in table 2.
Batch Reduction Tests
Batch-type tests were conducted over the temperature range 1,000° to 1,400° C. Their purpose was to determine relations among zinc extraction, time, and temperature as a guide for future continuous tests. An induction heating unit (0.45 MHz) was used to conduct these tests to allow rapid heating and cooling of the charge.
Reactants were contained in a graphite crucible which was heated by a graphite susceptor. Graphite wool insulation surrounded the susceptor, which was contained within a silica tube. This unit was enclosed in a chamber with vacuum connections and inlets for carbon monoxide, argon, and helium. The experimental procedure consisted of loading the well-blended 1:1 mole ratio charge of 2.00 grams reagent-grade ZnS and 1.14 grams minus 100-mesh iron powder in a ¾-inch-inside-diameter by 1-inch-deep graphite crucible, pumping the chamber down to approximately 1 x 10-¹ torr, and backfilling with CO to
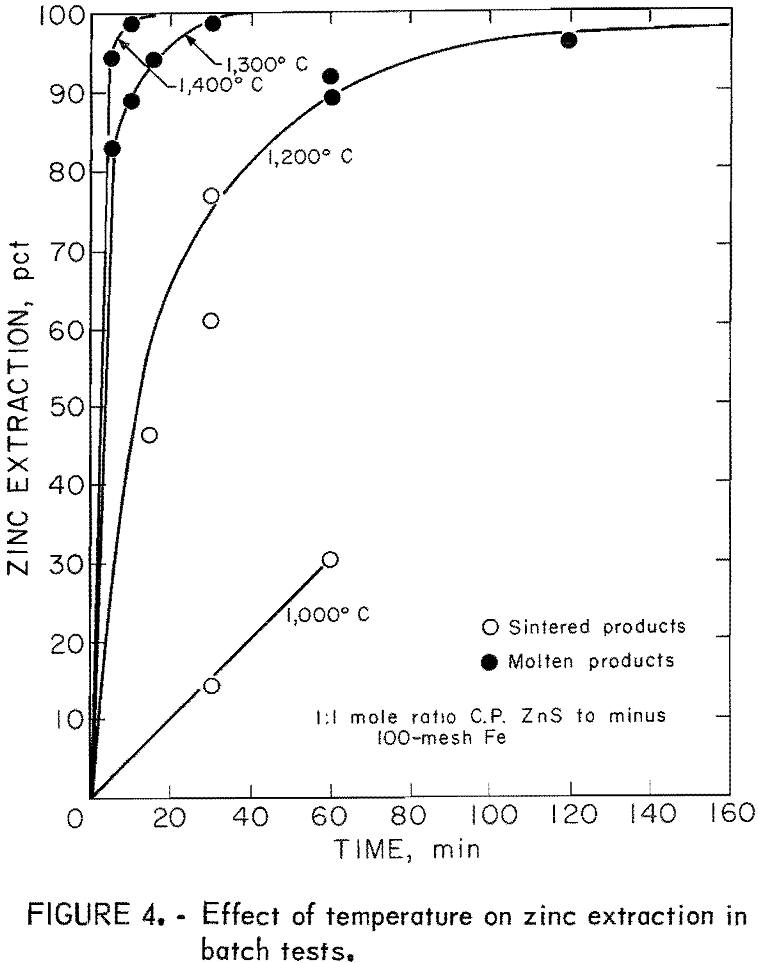
atmospheric pressure. Carbon monoxide was used rather than argon because with argon there was arcing caused by the high frequency of the heating unit. A small flow of CO was maintained throughout each run and monitored by observing the flow through a series of gas bubblers filled with concentrated H2SO4. The sample was heated quickly to the desired temperature and held for a set time; then the power was turned off. Immediately thereafter a flow of helium was directed at the bottom of the crucible to increase the cooling rate. The temperature within the graphite crucible was monitored continuously with a platinum versus platinum-10 pct rhodium thermocouple encased in a closed-end silica tube. Thermocouple interference from the radio frequency field was checked by turning the furnace off and on while at temperature. In most tests no interference was seen. When interference was observed, corrections were made during the run. A typical time-temperature recorder plot is shown in figure 3 as it appeared on the recorder. Heating rates above 800° C were approximately 200° C per minute. Cooling rates were slightly faster in this temperature range. The cold sample was removed from the furnace, weighed, and analyzed for zinc. Zinc extraction then was calculated based on the initial zinc in the charge and the zinc remaining in the matte. No attempt was made to collect the extracted zinc which condensed on the walls of the SiO2 tube as metallic zinc. No elemental sulfur was observed on the walls of the SiO2 tube or furnace. Figure 4 shows the zinc extracted as a function of time at 1,000°, 1,200°, 1,300°, and 1,400° C. Observation of the resulting FeS matte indicated that at about 85-pct zinc extraction the samples were completely molten.
At 1,300° C, 99-pct zinc extraction was attained within 30 minutes, while at 1,200° C, an hour and a half was required before 96-pct zinc extraction was attained.
These tests indicated that a liquid-state, reaction at temperatures greater than 1,200° C would be required to obtain satisfactory reaction rates and good extraction. From thermodynamic considerations, Kelley suggested a minimum temperature of about 1,300° C.
Semicontinuous Tests
The purpose of the semicontinuous test series was to determine the purity of zinc produced, to learn the distribution of the impurity elements Pb, Cd, Cu, and Fe between matte and zinc metal, and to point out any problems that might develop in feeding. Also, because municipal scrap could be used as reductant, possible problems with SO2 evolution and tin contamination of the zinc metal were investigated.
Since our batch work and the thermodynamic work of Kelley suggested a temperature of somewhere near 1,300° C, semicontinuous tests were made in the regions were liquid FeS would be formed. The equipment, which allowed continuous feeding of reactants and continuous condensing of zinc vapor, is shown in figure 5, and consisted of a graphite reactor of approximately 250-cm³ usable volume, a graphite condenser tube, and a stainless steel screw feeder. A flow of protective CO was introduced into the barrel of the screw feeder. In a typical test, the reactor was heated to within 100° of the desired temperature, and feeding of the charge (a well-blended dry zinc concentrate plus electrolytic iron chips) through the screw feeder was begun. When the feed reached the end of the screw, the temperature of the reactor was at test temperature. Most tests were 1 hour long, not including the holding time. Plugging of the feeder occurred on some tests owing to the sintering of the charge. This problem was corrected by water cooling the barrel of the feeder. The reactor was heated with a split graphite element, and the power input was controlled manually. The temperature was monitored with a tungsten- 5 pct rhenium versus tungsten-26 pct rhenium thermocouple, the output of which was recorded continuously.
The reactor was dismantled after cooling, and the matte was removed from the crucible, weighed, and analyzed. The very small amount of slag phase present was included in the matte. Zinc metal was removed from the condenser, weighed, and analyzed. A constant feed rate of 1 lb/hr was attempted for all tests; however, this was not always maintained owing to differences in concentrate texture. Actual feed rates ranged from 0.62 to 0.9 lb/hr. To insure that the last feed had sufficient time to react, a number of tests were run with different holding times. The results of these tests indicated that 30 minutes was sufficient time for reaction products to reach equilibrium.
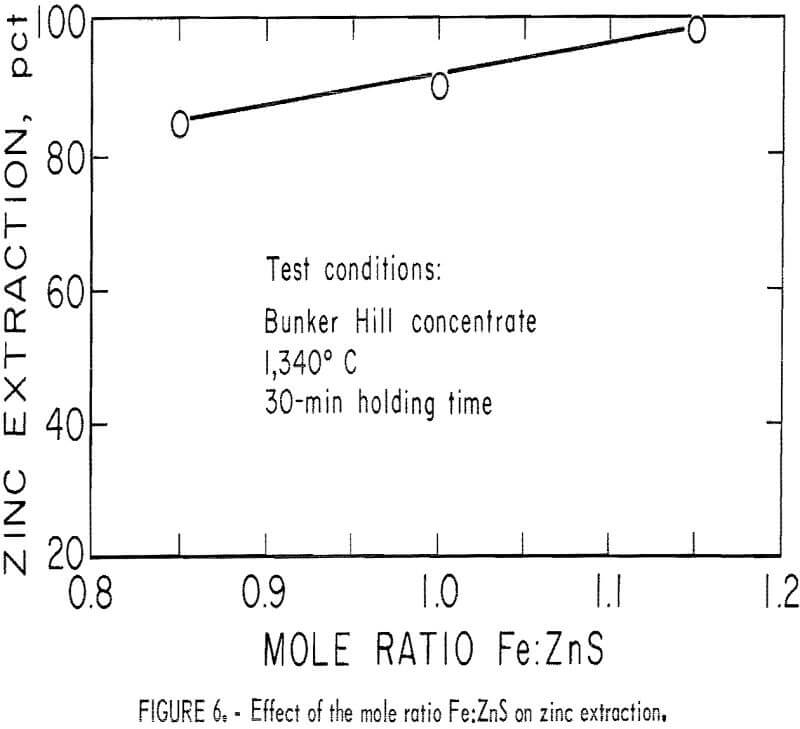
The effect of temperature on zinc extraction in these tests was found to substantiate both the theoretical and batch work. A temperature of approximately 1,300° C was needed for good zinc extraction. The relation between the mole ratio Fe:ZnS and zinc extraction is shown in figure 6. The 1.15 mole ratio approximately equals the ratio 1.22 which is required to satisfy equilibrium requirements at 1 atmosphere zinc vapor pressure and where a zinc extraction is estimated to be 93 pct. Zinc extraction of 98 pct, indicated by our work, is not surprising because the zinc vapor pressure over the system was less than 1 atmosphere, which would enhance zinc extraction from the matte.
![]()
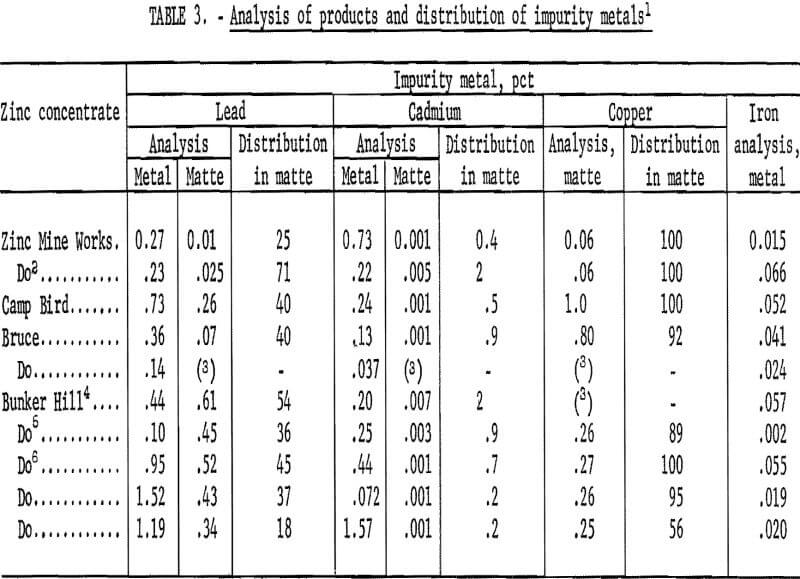
A series of tests was run using the four zinc concentrates to determine the distribution of the impurity elements Pb, Cd, Cu, and Fe between matte and zinc metal. The tests were conducted at 1,340° C with a 1:1 mole ratio of Fe to ZnS, with the matte being held at temperature 30 minutes after cessation of feeding. Both matte and zinc metal were analyzed in most cases. The matte was crushed to minus 80 mesh before analysis, and the samples of zinc were selected at random from the solidified zinc metal ingot.
Analytical results are reported in table 3. Condensation in the tube -type condenser was decidedly nonuniform; hence, the matte analyses provide more reliable distribution data for the volatile elements. Lead was distributed slightly in favor of the zinc metal. Analyses are scattered but tend to cluster around a 60-pct distribution in the zinc metal. Cadmium was vaporized quantitatively from the matte but was not collected completely in the zinc. Copper was collected in the matte with good accountability. Iron in the zinc metal ranged from 0.002 to 0.066 pct. Most of the zinc produced was within Prime Western specifications.
Municipal scrap, a likely source of low-cost iron for the reductant, would be contaminated with low levels of tin. Two tests were run using a feed simulating municipal scrap containing elemental tin (minus 100-mesh) equal to 2 wt-pct of the iron. This level is an order of magnitude larger than usually encountered in municipal scrap. Test conditions were 1,340° C, Fe:ZnS = 1, and a 30-minute holding time. Both metal and matte were analyzed, and tin was not detected at the 0.05-pct level in the metal. The matte contained 0.55 pct tin. On the basis of this analysis, approximately 45 pct of the tin was not accounted for. No attempt was made to account for this apparent loss.
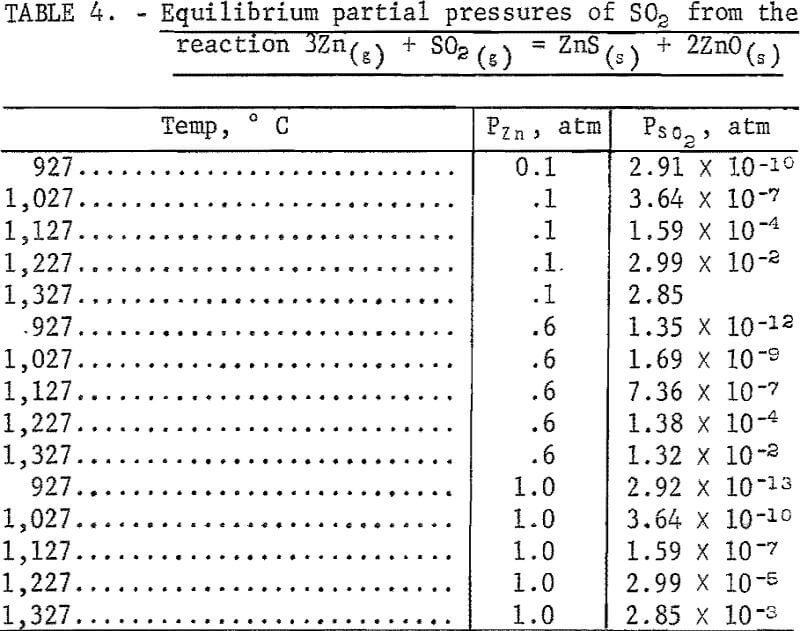
Two additional tests were made using Zinc Mine Works concentrate to simulate feeding of the magnetic, fraction of incinerator residue which would contain considerable rust. The reductant comprised electrolytic iron chips wherein 5 and 20 at pct of the iron was replaced with Fe2O3 powder. Test conditions were the following: 1,340° C, Fe:ZnS = 1, and a 30-minute holding time. The temperature at the discharge end of the condenser tube was approximately 200° C. Samples of the offgas were taken at the exit of the condenser. Sulfur oxides were not detected at the 0.01-pct level in any of the tests. The absence of SO2 in the exit offgas is probably due to back reaction with zinc vapor: 3Zn(g) + SO2(g) = ZnS + 2ZnO. Table 4 shows the partial pressures of SO2 obtained from the above reaction, assuming the activities of ZnO and ZnS are unity.
Conclusions
The reduction of zinc sulfide concentrates with iron has been demonstrated to be technically feasible on a semicontinuous bench-scale operation. A temperature of approximately 1,300° C was satisfactory for obtaining good zinc extraction. Contained copper reports to the matte product, whereas cadmium reports to the zinc metal. Lead is distributed about 40 pct in the matte and 60 pct in the metal. When tin was added with the iron to simulate tin-bearing scrap, the majority of tin was found in the matte while no tin was detected at the 0.05-pct level in the metal. When up to 20 pct of the iron reductant was replaced with Fe2O3, SO2 gas was not detected in the condenser offgas.
Larger scale tests are needed to determine energy consumption and air pollution problems. Disposal of the FeS should pose no problem since a commercial process has been used for several years in Europe for the treatment of pyrite cinders.

