Table of Contents
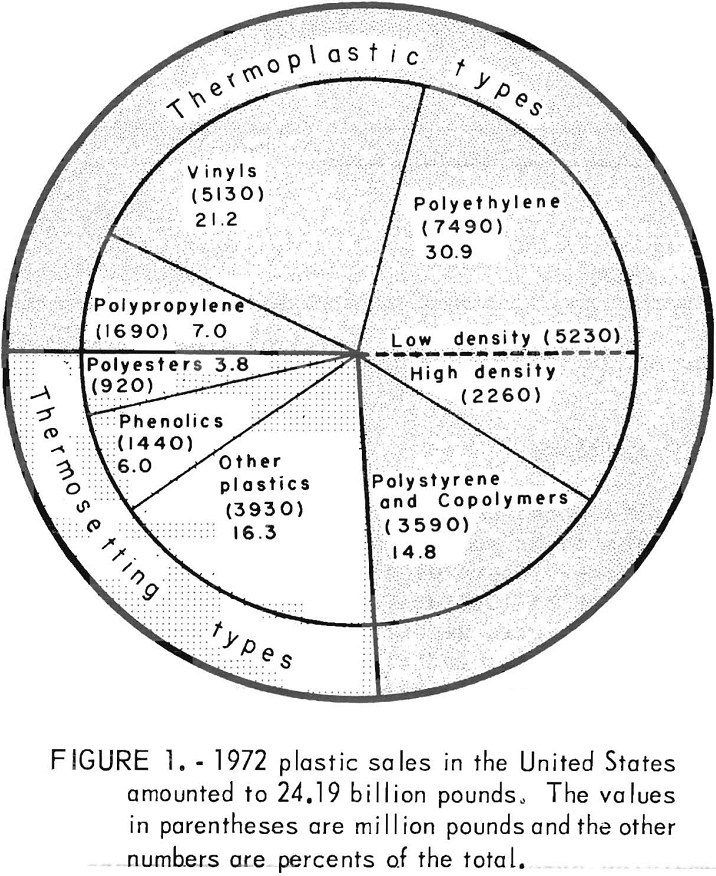
This is the age of plastics. Plastics are rapidly replacing metal, glass, and wood in parts of appliances, automobiles, prepackaged consumer goods, pipe, beverage containers, and furniture. The total sales of U.S. plastic for 1972, as shown in figure 1, was 24.19 billion pounds versus 19.9 billion pounds during 1971, an increase of over 20 percent. The breakdown in figure 1 shows that plastics come in two basic types, thermoplastics or thermosets. Thermoplastics are varieties of polymeric materials which become soft when heated and can be molded, extruded, and shaped by various techniques, In contrast, thermosetting or chemically hardened plastics do not soften upon heating and cannot be remolded. The most common thermoplastics are as follows:
- Polyolefins, including high-density polyethylene (HDPE), low-density polyethylene (LDPE), and polypropylene (PP).
- Styrenes, including polystyrene (PS) and acrylonitrile-butadiene-styrene (ABS).
- Vinyls, primarily polyvinyl chloride (PVC) and polyvinylidene chloride (PVDC).
The United States generates about 300 million tons of urban waste per year of which about 160 million tons is collected. This collected urban refuse reportedly contains 2 percent plastic. Discarded packaging accounts for 60 percent of all plastic found in disposal areas.
The disposability of plastics has been and is the concern of several investigations. Waste plastic can be disposed of by incineration, landfill, or recycling. Incineration of vinyl-containing plastics yields an atmosphere containing toxic hydrogen chloride which readily forms corrosive hydrochloric acid. It has been stated that this will not create a serious corrosion problem in a properly designed and operated incinerator; however, over 70 percent of all U.S. incinerators are designed and operated inadequately with respect to this problem. On the plus side, heterogeneous plastic waste after the removal of the vinyls could serve as a source of high-energy fuel since the heats of combustion of PE, PP, and PS are in excess of 16,000 Btu/lb.
The Federal Bureau of Mines regards the 300 million tons of urban refuse generated in the Nation each year as “resources out of place.” A portion of the Bureau’s research has been directed toward methods of separation, recovery, and recycling of metal-, mineral-, and energy-based materials in urban refuse. For instance, it has been shown by Bureau scientists and engineers that municipal incinerator residues can be processed at costs of $1.80 to $4 per ton to yield concentrates of metals, glass, and minerals worth $22 per ton—materials that are currently being deposited in landfills in many cities. A corresponding system is presently under development to recover ferrous and nonferrous metal, glass, paper, and plastics from raw refuse.
Others have devised systems for recycling portions of materials from raw refuse such as the wet processing system designed by the Black Clawson Co. A 150-ton/day demonstration plant was constructed under a grant from the Bureau of Solid Waste Management and is presently operating at Franklin, Ohio. A recent review of the state of development of resource recovery systems has been published. Most of these systems stress the recovery of paper, metal, and glass, or the recovery of fuel values.
The plastics industry is an important and growing part of our Nation’s economy. The conservation and reuse of waste plastic is especially important during this time of energy crisis. The fact that plastics come from petroleum increases the need for their recycling and reuse, because plastic fabricators are currently experiencing difficulty in obtaining resins owing to a lack of petroleum feedstocks. Although manufacturers of thermoplastic products have for years been recycling trimmings, runners, sprues, and rejects generated during production, very little if any post-consumer plastic scrap is reused. With the development of urban-refuse processing systems having the capability to produce plastic concentrates, the feasibility of reusing thermoplastics with or without separation into generic types should be considered. Industrial sources of waste plastics, such as that generated by the secondary metals industry during the reclamation of copper and aluminum from insulated wire scrap, would require similar separation research before its reuse potential can be predicted.
The major objective of this research was to show that heterogeneous thermoplastic concentrates from urban and industrial refuse could be separated into reusable generic plastic types. The optional methods for separating plastics into major types are essentially those based on shape, density, selective dissolution, and electrical properties. A separation scheme using different density solutions suggested during this research investigation has been described previously. A large portion of the results reported in this paper are based on a sink-float-elutriation separation process which was developed and applied to the separation of urban and industrial plastic wastes. Methods for utilizing these reclaimed secondary thermoplastics, such as hot pressing, injection molding, extrusion, and thermal decomposition, were also investigated.
Experimental Separation Procedures
Air Classification
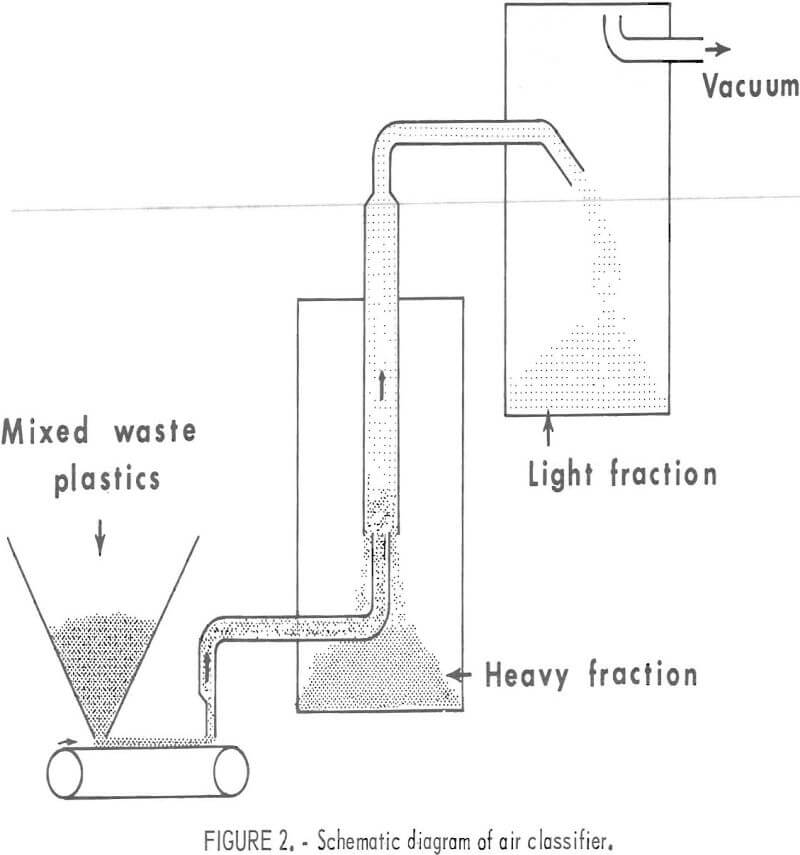
A large portion of collected waste plastic such as film, fiber, and foam can be separated from the molded plastic and container fragments by air classification. Numerous experimental and commercial types of air classifiers are available. An inexpensive one, powered with a household vacuum cleaner, was designed and built in the Rolla laboratory, The design is shown schematically in figure 2. This vertical air classifier is capable of classifying 50 to 75 pounds of mixed plastics per hour. The air velocity in the 3-inch-diameter vertical column is approximately 500 to 750 ft/min during operation.
Liquid Media
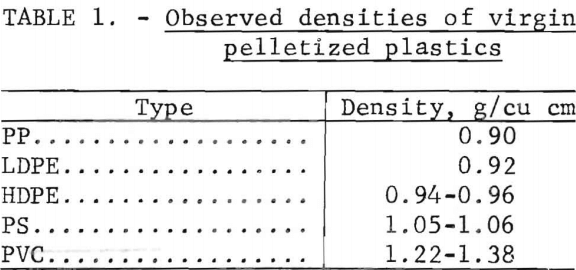
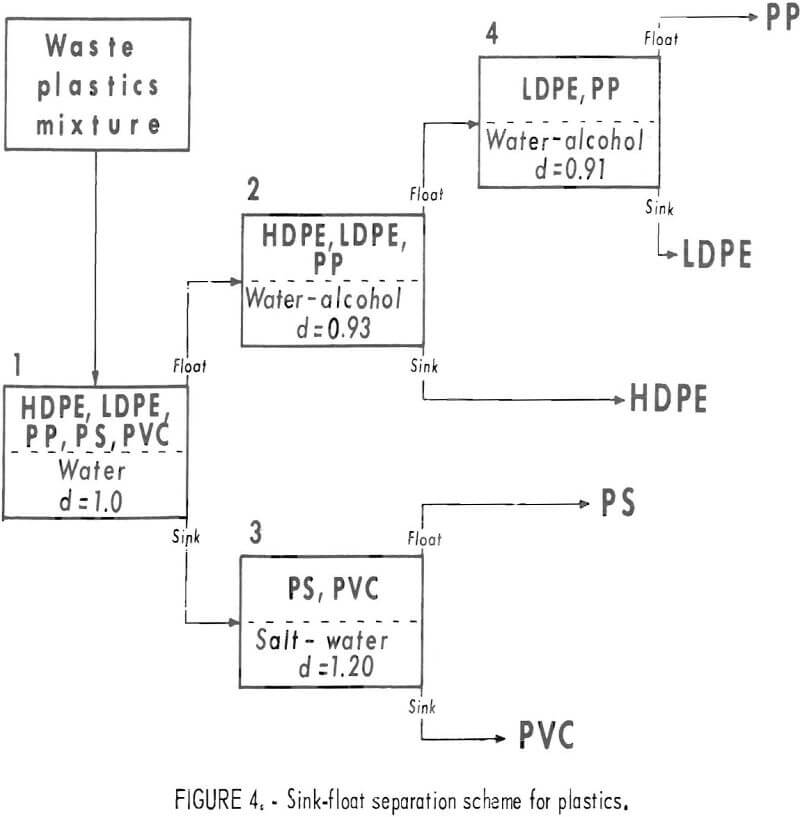
The densities of virgin thermoplastics are nearly the same as that of water, and vary in a manner that favors gravity methods in aqueous solutions for separating them by types. Measurements of the densities of various pelletized virgin plastics gave the data in table 1. As can be seen from these data the polyolefin family (LDPE, HDPE, PP) of materials has a density range from 0.90 to 0.96 g/cu cm, PS compounds 1.05 to 1.06 g/cu cm, and PVC compounds 1.22 to 1.38 g/cu cm. Although additives may alter these densities, laboratory results on plastic waste have confirmed that most scrap plastics are in the density ranges given in the table. Experimental separations of the five major types of plastic from wastes were conducted in unagitated vessels with distilled water, a calcium chloride solution, and two alcohol-water combinations (fig. 3). These media gave virtually complete separations of hand-sorted waste plastic samples. Based on this sink-float method, a theoretical scheme of separation for the five most plentiful components of waste thermoplastics in packaging wastes was devised and is given in figure 4.
This scheme relies on the use of fluids differing in density and would entail practical requirements for rinsing, waste disposal, and control of liquid density.
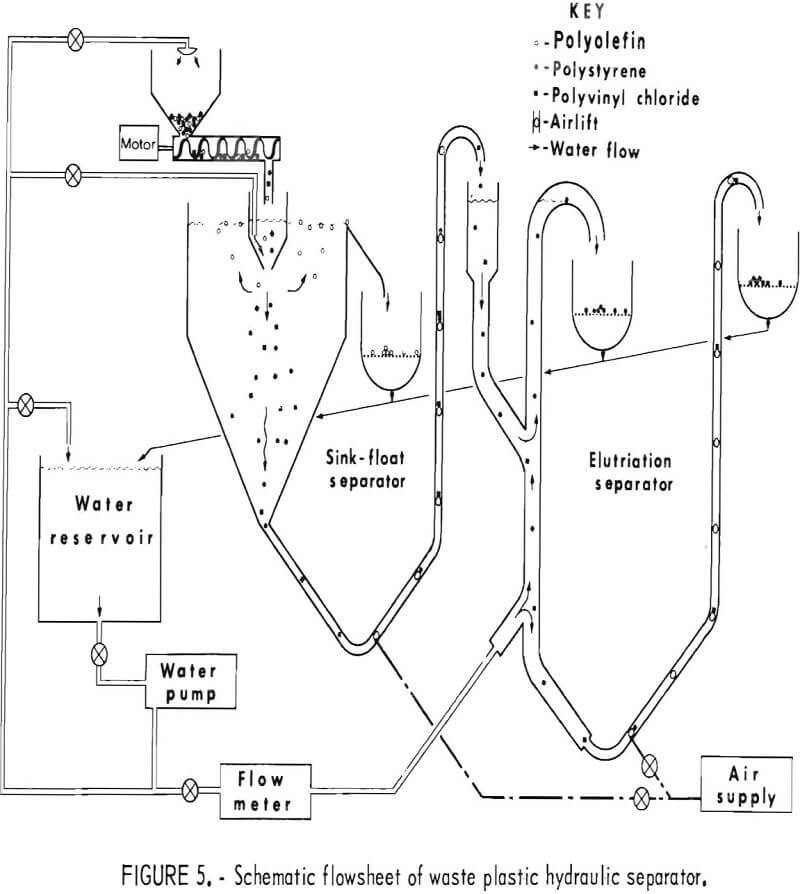
It was deemed desirable that a separating system be devised which would use only water as the parting medium. A small hydraulic separator was designed and built for separating chopped waste plastics into three fractions: polyolefins, polystyrene, and polyvinyl chloride. The schematic diagram of this separator, a combination sink-float and elutriation unit, is shown in figure 5. Mixtures of chopped plastics were fed into the sink float separator where the polyolefins were floated off and the other components of the mixture

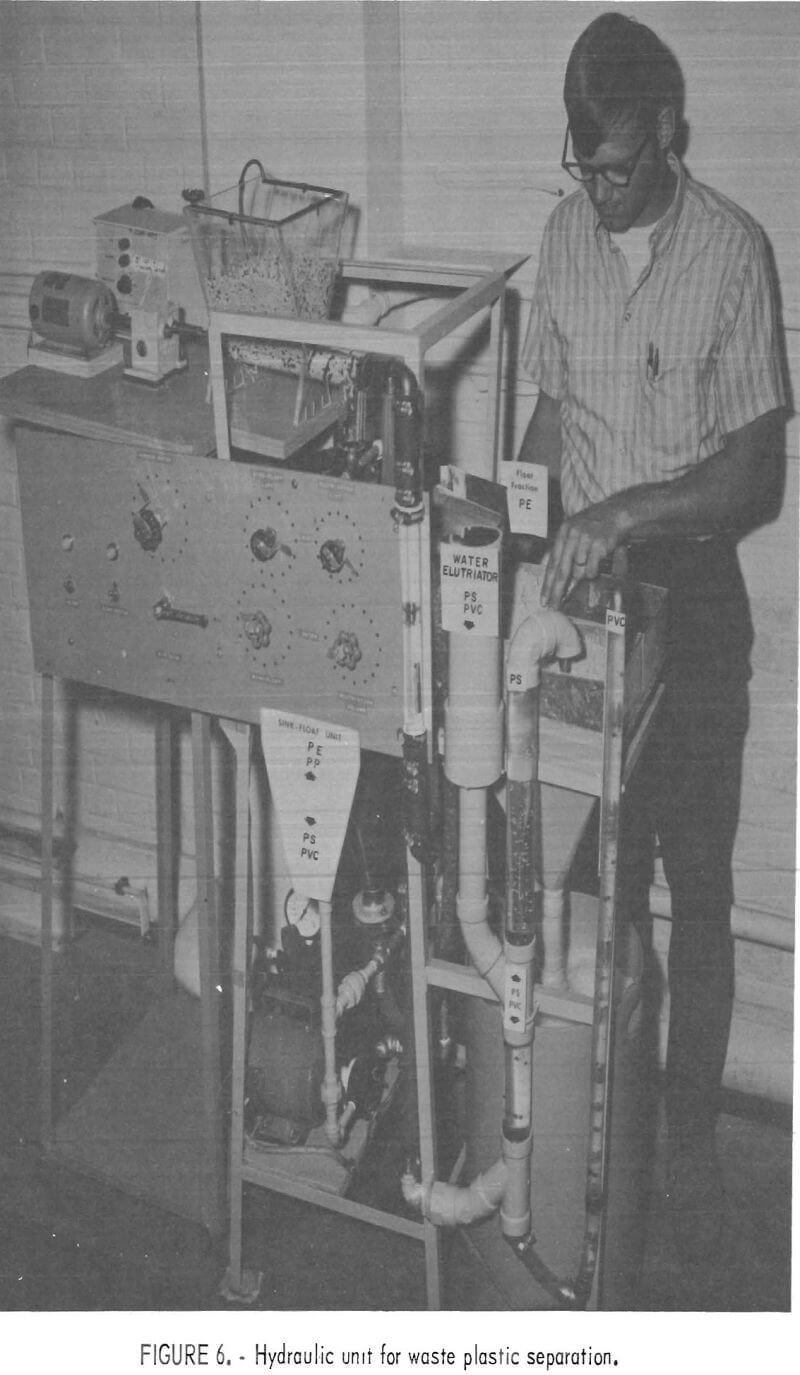
sank. The sink portion was transported via airlift to the elutriation column where the PS was carried out the overflow and the PVC sink fraction was air lifted into the underflow receptacle. The segregated fractions were all caught on screens while the water was recycled to the main reservoir. The laboratory model shown in figure 6 has been used successfully to separate mixtures of virgin pelletized plastics and chopped waste plastics at rates from 5 to 50 pounds per hour. An important consideration in separating plastics by hydraulic methods was the addition of 0.3 cc surfactant per gallon of water.
The performance of the laboratory model hydraulic separator has been successful enough to recommend it as a prototype design for larger units capable of processing the plastic waste output of municipal waste treatment plants.
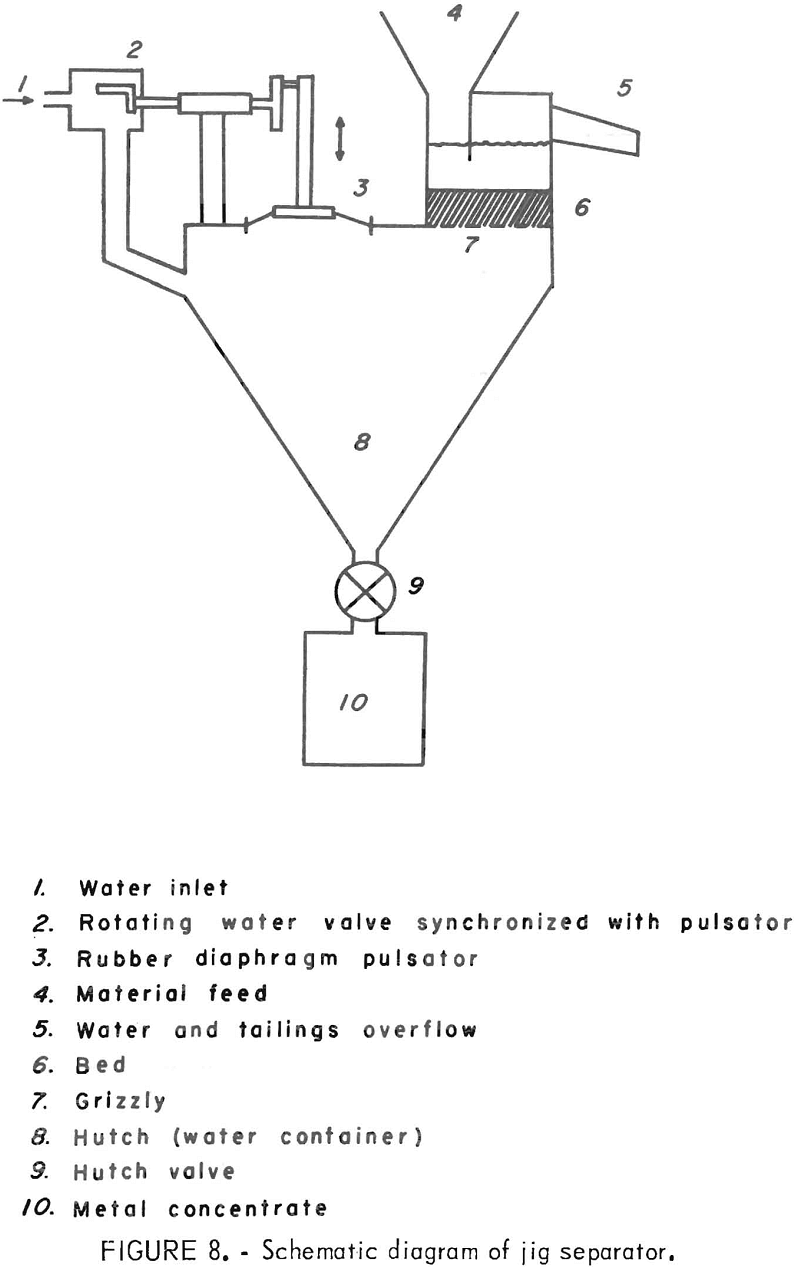
A method which has been used successfully to reduce the residual metal from waste wire insulation tailings is the mineral jig. In this unit the mixed waste material is fed onto a pulsating liquid-solid bed. As the liquid
is pulsed, the gravel bed is forced apart repeatedly, allowing the heavy material (metal) to sink through the bed and eventually through the slotted screen at the bottom into a collecting chamber. At the same time, water fed into the unit carries off the lighter material (plastic) in the overflow. The jig and a schematic diagram of its operation are shown in figures 7 and 8.
Screening and Electrostatic Separation
In some instances vibrating screens can be used to concentrate or remove a specific component in mixed waste. Such is the case with wire insulation tailings. The residual metal wire, in most cases being of smaller size than the insulation, can be concentrated by screening. This method can be used to upgrade the metal content prior to metal recovery by processes such as jigging or electrostatic separation.
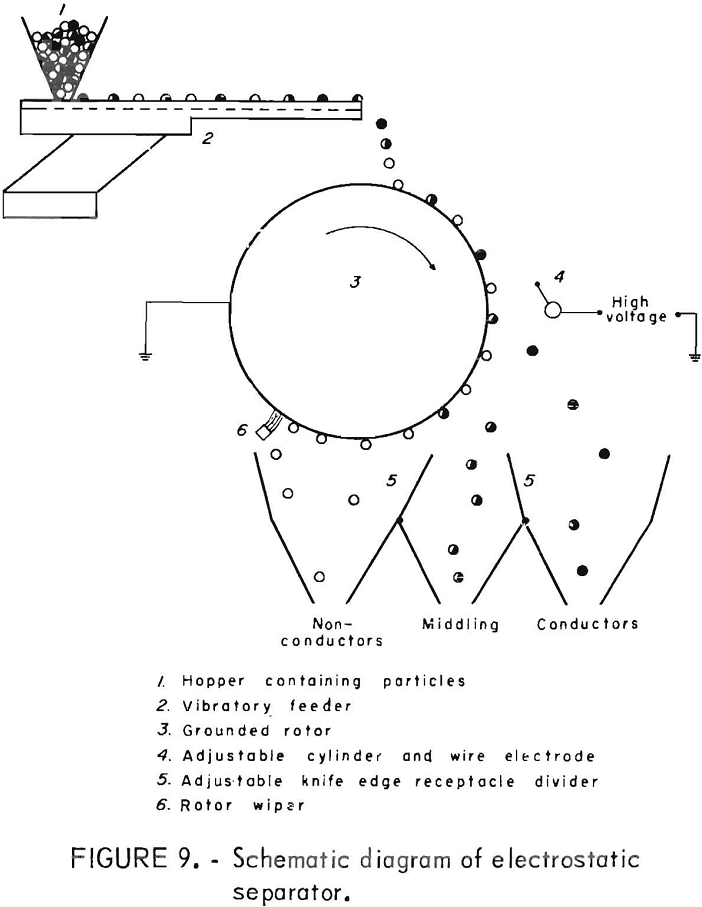
In an electrostatic drum-type separator, the material is fed onto an electrically grounded drum which is rotating in a highly charged electric field. The plastic is pinned to the drum and removed by a fixed wiper blade and the wire is thrown into another compartment. Adjustable compartments are available to receive the products, and the material in the middle compartment may be rerun for additional separation. Figure 9 shows a schematic diagram of the operation of the electrostatic separator. The electrostatic separator has been used with some success to separate plastic film and styrofoam.

Experimental Results
Local Plastics Collection
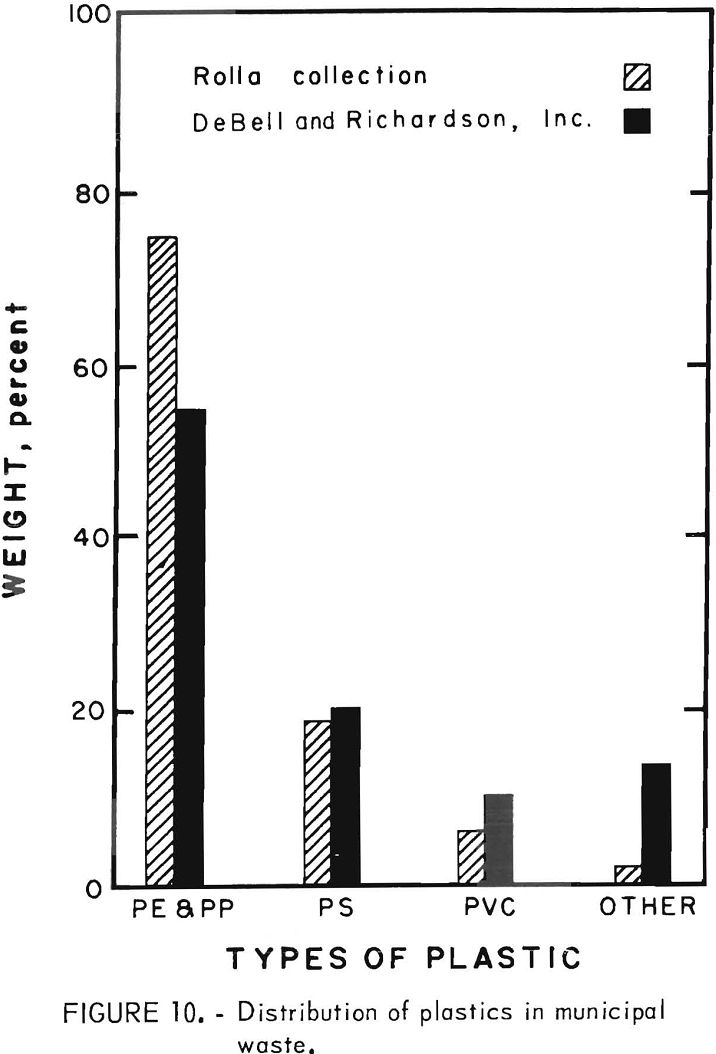
The initial experimentation on waste plastics was performed on an 800-pound sample of household plastic waste collected during a special 1-week community-wide collection drive in Rolla, Mo. The distribution of plastic types in the community collection was analyzed by hand sorting half of the collected sample into four classes. The bar chart (fig 10) shows the comparison of the community-collected plastic to the distribution of the plastics in solid waste collected in the United States in 1969 according to an estimate made by DeBell and Richardson, Inc. It can be seen from figure 10 that 85 to 98 percent of the plastics in solid waste falls into the three major types of thermoplastics. This locally collected plastic waste was primarily packaging waste and, therefore, probably biased in favor of blow-molded bottles made of polyethylene. Nearly 2,000 bottles were in the collection, about half of which were detergent containers. The balance was about evenly divided between cosmetic, food, and medicine containers. The collected plastic had a bulk density of about 0.04 g/cu cm. After chopping to minus ½-inch size the bulk density was increased by a factor of 10.
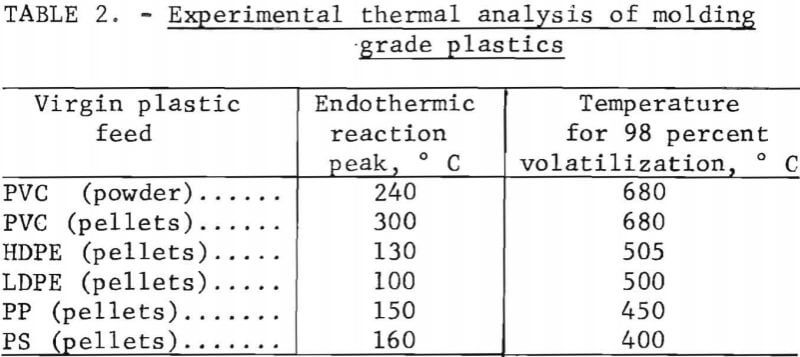
Several methods were used to hand-sort the plastics from the collection into types based on feel, sound, appearance, flame behavior, and odor. For example, polyethylene feels greasy and is usually somewhat flexible, whereas polystyrene is brittle and is noisy when dropped or flexed. A scheme of analysis by flame behavior and odor was especially useful for chlorinated compounds. A hot copper wire touched to PVC and then placed in the blue flame of a gas burner produces a bright green hue. Differential thermal analysis (DTA) and thermogravimetric analysis (TGA) were used where simpler methods failed; the exothermic and endothermic reaction temperatures give clues to polymer identity. Table 2 gives the experimental results obtained in the laboratory on several virgin plastic samples, illustrating the values that were used to classify samples of unknown plastic type.
Experimental work on the collected waste plastic has produced some guidelines for systematic methods of processing. The first step, as already mentioned, was chopping the material to uniform size. This was done in a rotary knife-bladed granulator, equipped with interchangeable screens with hole sizes of 1/8 inch to ½ inch. The chopped material was then air classified to remove the plastic film, foam, dust, and unattached paper. In some cases this process might be accomplished during granulation. During air classification, about one-fourth of the material was removed in the air-light fraction. The air classifier was shown in figure 2. The next process was that of cleaning the air-heavy fraction to remove contaminants such as dirt, labels, adhesives, and residual contents. A number of washing solutions and agitation methods were tried. The best results were obtained by using a 1-normal solution of sodium hydroxide containing 150 grams of sand per liter of solution in an attrition scrubber. Approximately 98 percent of the particles were clean after a 1-hour period of this washing treatment. Tap water, containing the same amount of sand, cleaned 96 percent of the particles and would be more practical on a large scale in view of the cost of adding and purifying the reagent for reuse.
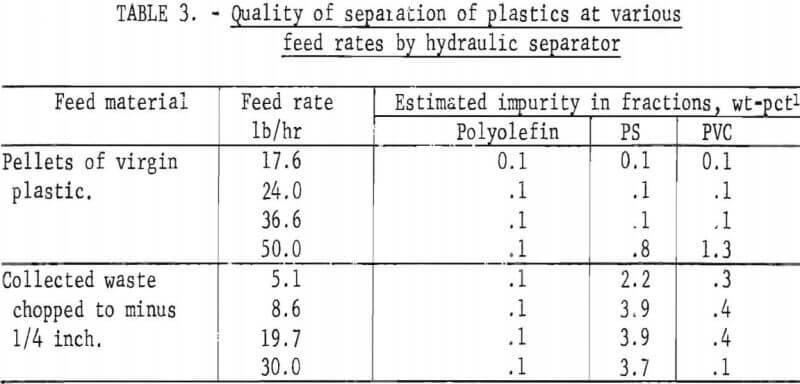
The final operation was that of separating the air-heavy cleaned plastics into major types. This was accomplished using the hydraulic separator which was described earlier and shown in figures 5 and 6. Three fractions were produced from this separator: (1) the float fraction which contained polyolefins (PE and PP), (2) the elutriator overflow (PS), and (3) the elutriator underflow (PVC). A sink-float analysis was performed on fractions obtained at various feed rates. These data are presented in table 3. In most cases, over 96 percent of the material reported in the correct density level.
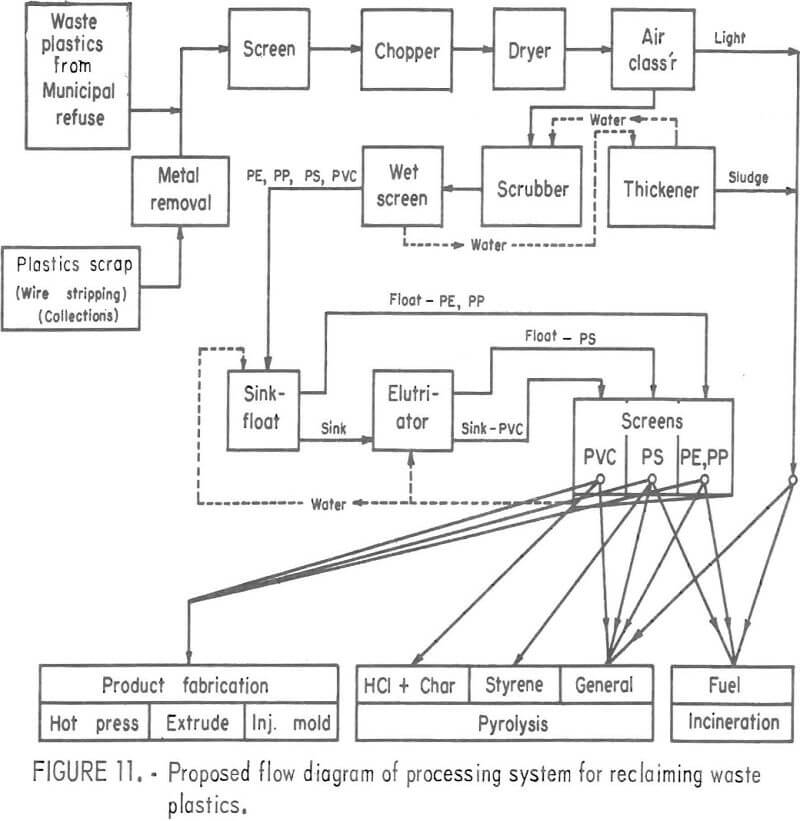
A processing system based on the above unit operations has been proposed and is shown in figure 11. This system would conceivably operate in conjunction with a raw urban refuse processor which would provide a plastic concentrate fraction for the feed material. The proposed system would further upgrade the plastics fraction and concentrate three major types of plastics for selective reuse by one of several methods. Unit operations of the type used for minerals processing would be utilized in a system of this type
Recycling Center Product
A sample of plastic waste collected at the Wellesley, Mass., recycling collection center was separated into primary plastic types. The as-received material had been chopped to minus 1/4-inch size and the metal lids, etc., removed by hand prior to chopping. A 2,500-gram sample was attrition scrubbed twice in 10 liters of hot tap water (50° to 60° C) containing 150 grams of sand per liter of water, for 30-minute periods. The sample was then rinsed with water and oven dried at 85° C. Approximately 20 percent of the material was less than 10 mesh in size and was removed by screening prior to hydraulic separation. The balance of the material was separated in the
hydraulic separator unit. Results of the separation and sink-float analysis of each fraction are shown in table 4. As can be seen from the table, a very high percentage of this waste material was polyolefin. A waste of this type containing a large amount of PE was reused after washing and separation.
Pilot Plant Concentrates
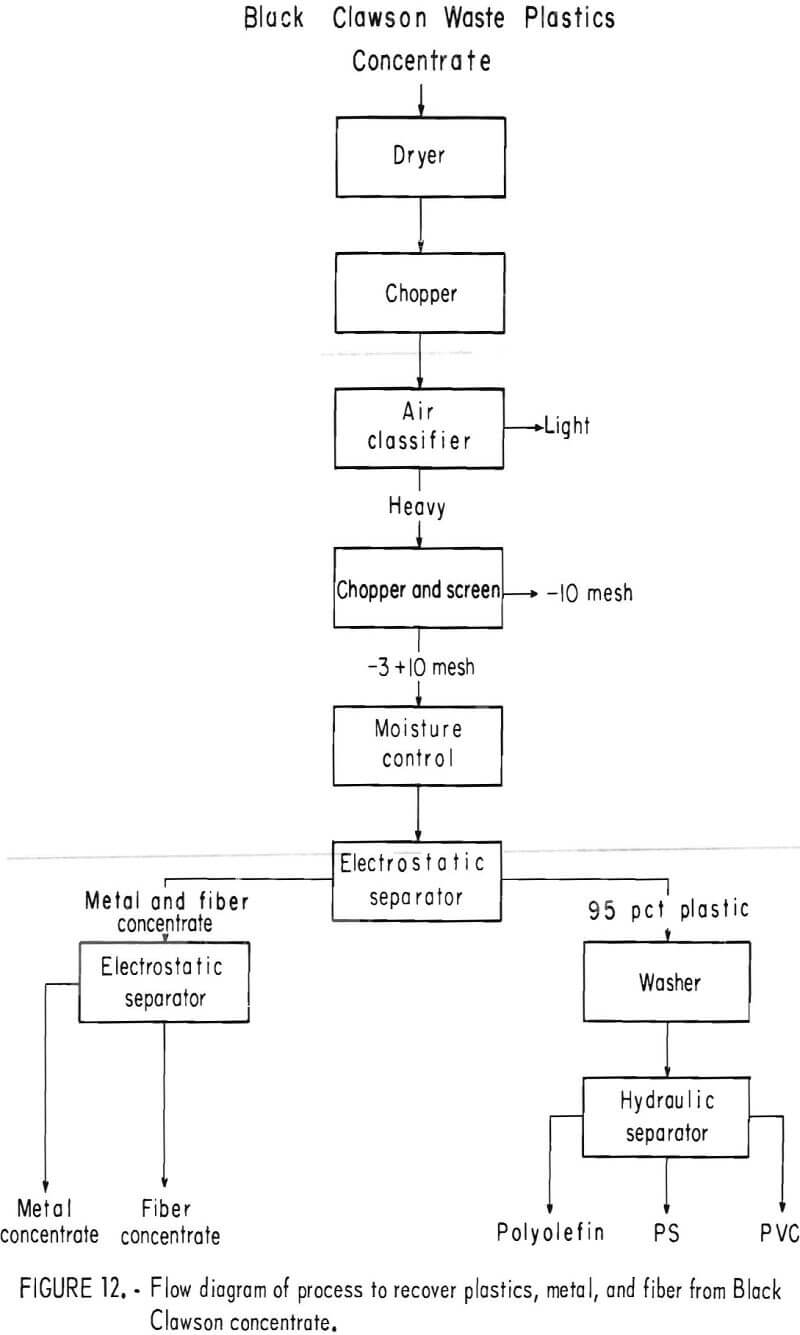
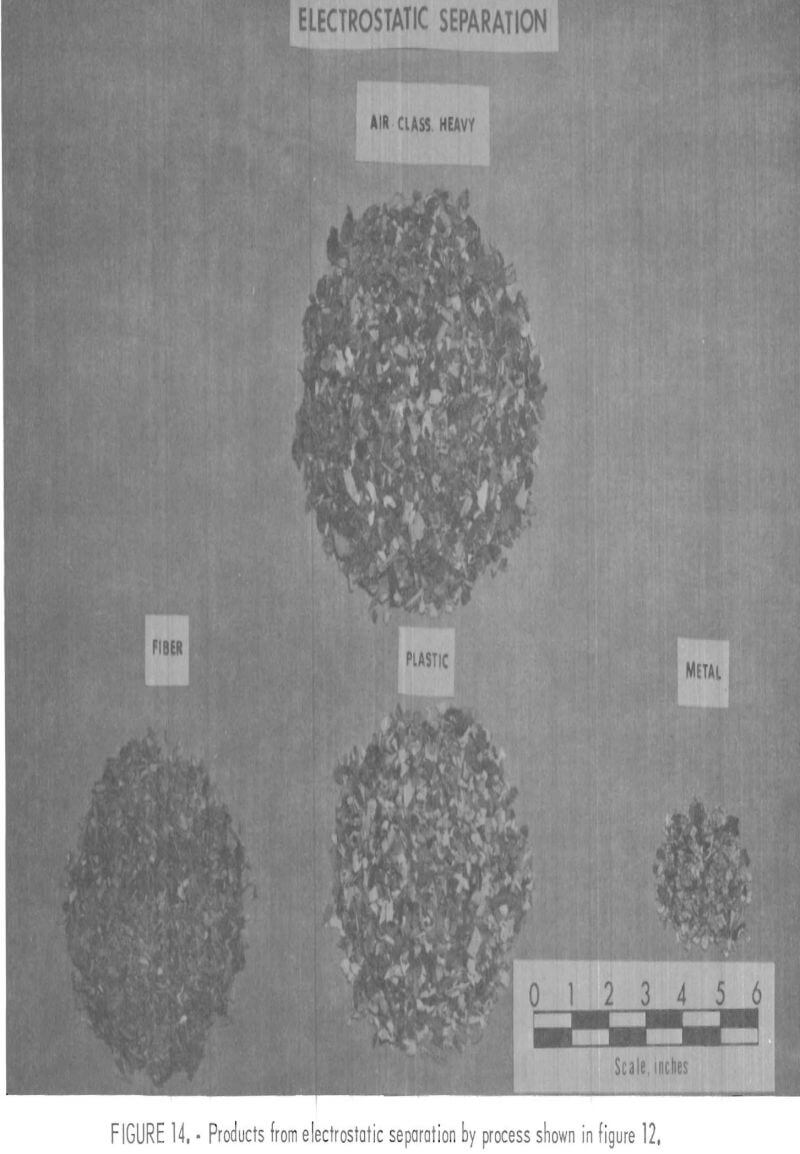
A plastic-rich sample from the Black Clawson FIBRECLAIM Urban Refuse Recovery System, at Franklin. Ohio, was obtained for upgrading and separation study. According to Black Clawson, the sample had been upgraded from 2.5 percent plastic by repulping and screening. This sample represented 13 percent of the refuse received at the Black Clawson plant. Oven drying and petrographic examination showed that the material contained 55 percent moisture, 14 percent plastic, 0.6 percent metal, and 30.4 percent mixed fiber, wood, glass, etc. The dried sample was chopped to minus ½-inch and air classified. The 25 weight-percent air-heavy fraction contained 45 percent of the total plastic in the dried sample. During air classification, approximately 55 percent of the total plastic reported in the air-light fraction. It contained primarily fiber and film. Upgrading the air-heavy fraction to 95 percent plastic was accomplished by chopping and screening the material to minus 3- plus 10-mesh size and then concentrating by electrostatic separation. In the first electrostatic separation, 8 to 10 percent moisture was necessary to obtain the best separation of plastic from the balance of material. The thrown fraction, containing the metal and fiber rejected in the first electrostatic separation, was dried and then separated further electrostatically. The metal concentrate from the second pass contained 75 percent metal, mostly aluminum foil, which amounted to a 60-percent recovery of the metal from the original sample. This procedure recovered 99 percent of the plastic contained in the air-heavy fraction. The total plastic recovery from the original sample was approximately 45 percent owing to the loss of plastic film to the air-light fraction. The recovered plastic was then attrition scrubbed in hot tap water (50° to 60° C), containing 200 grams sand per liter of water, for a 1-hour period and then was rinsed with tap water. After the scrubbing treatment it was apparent that the plastic was not entirely clean, although most of the loose dirt and attached paper had been removed. The final separation of the plastics into major types was achieved by hydraulic separation with the unit previously described and shown in figures 5 and 6. Sink-float analyses of the hydraulically separated fractions are shown in table 5. It can be seen from the table that the polyolefin and PS fractions are relatively uncontaminated compared with the PVC fraction. It is understandable that the lowest purity fraction would be PVC, because most of the thermosets and composites would fall in the PVC density range. The overall process, for the recovery of plastics, metal, and fiber, is shown in the flow diagram, figure 12. Figures 13, 14, and 15 show the raw sample and the relative quantity of products from the application of the process shown in figure 12 to the Black Clawson sample. In figure 15, the float fraction from hydraulic separation is labeled PE. This should technically be called polyolefin, but since the major type of plastic in this fraction is PE, it is so designated.


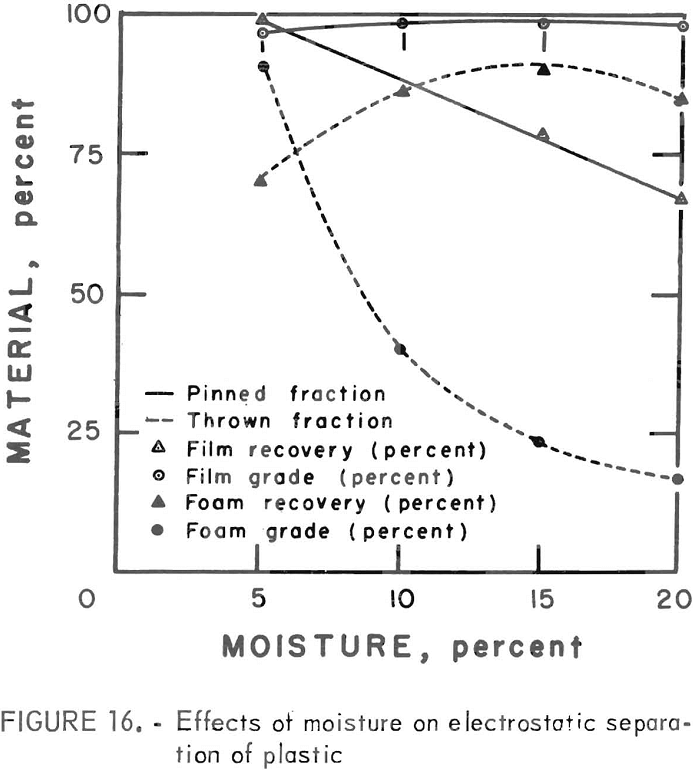
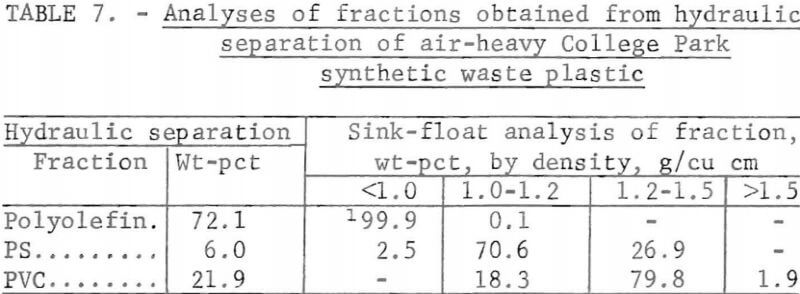
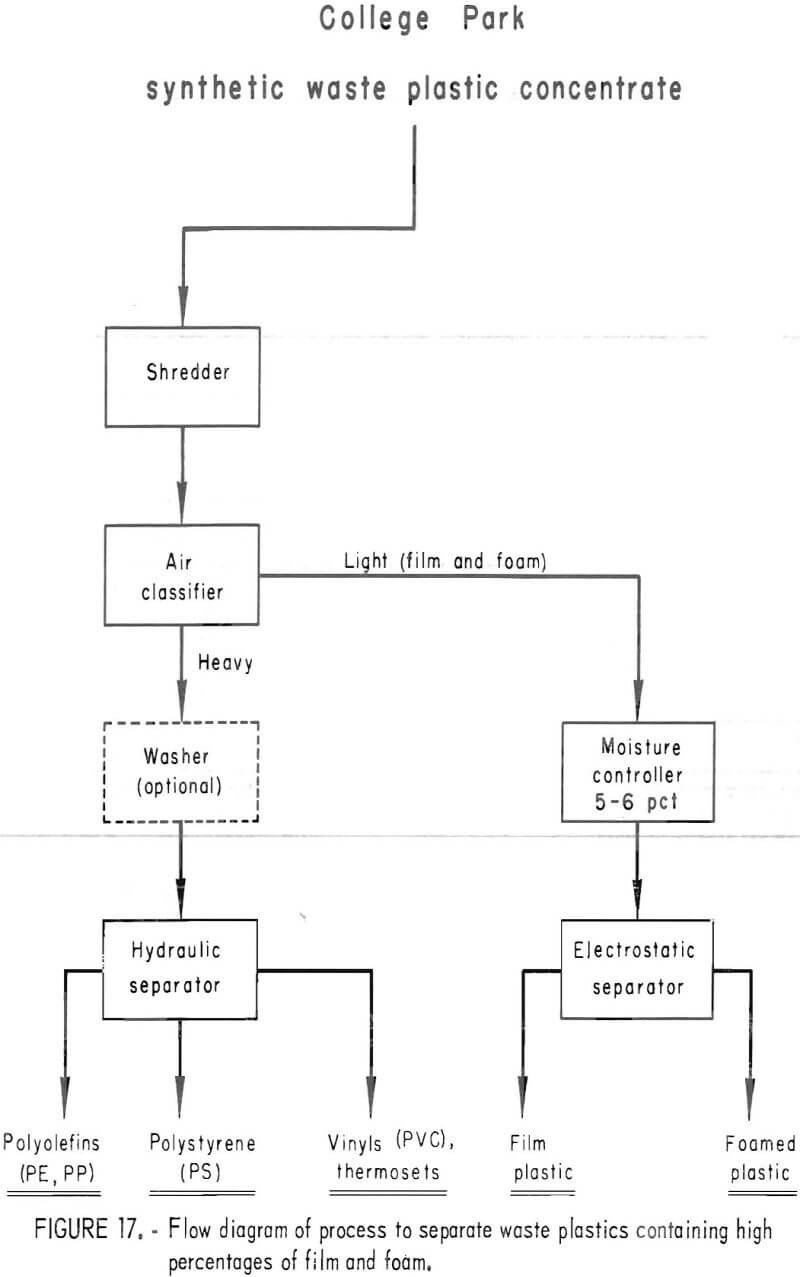
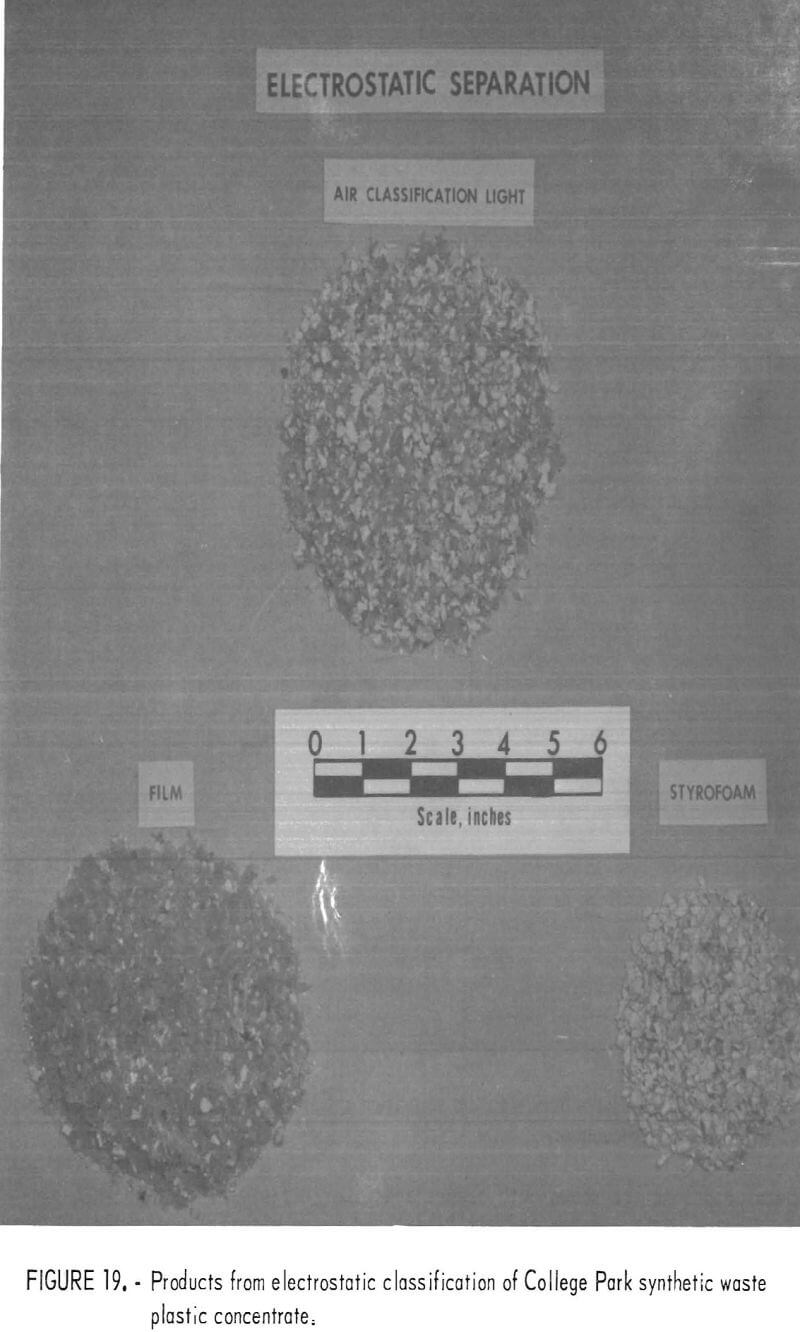
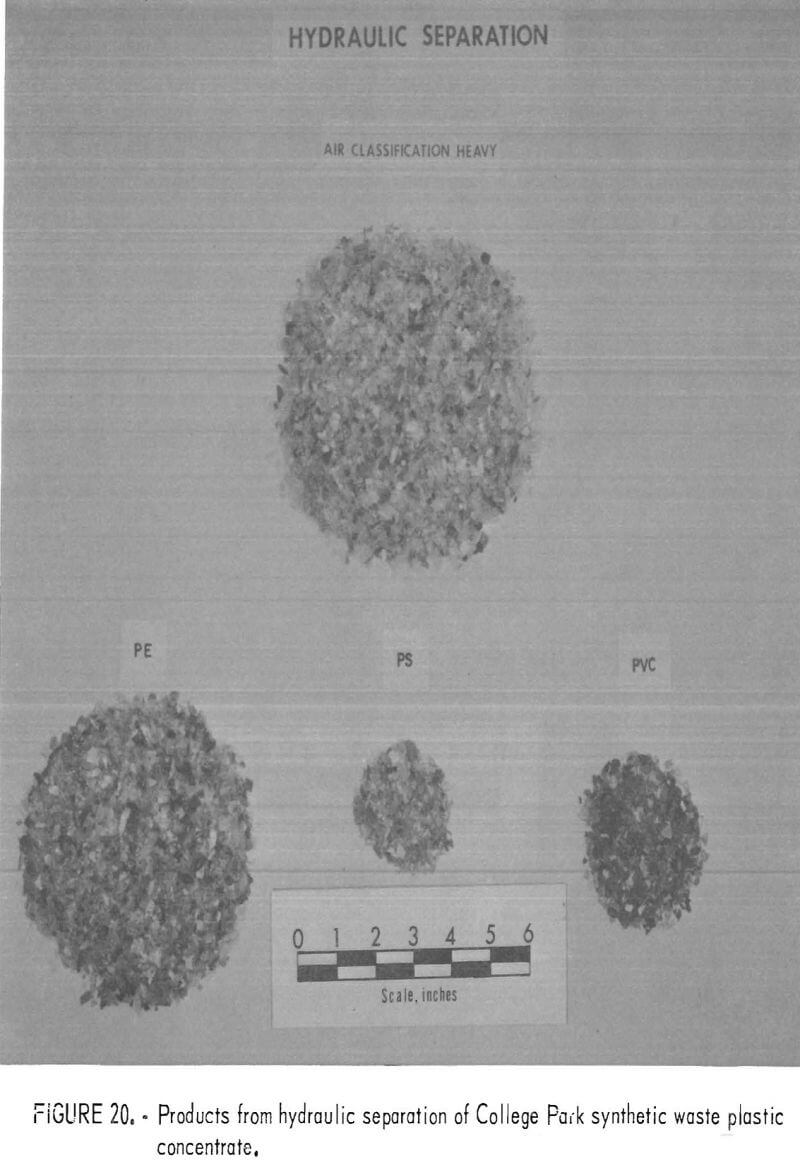
A synthetic waste plastics concentrate sample was received from the Bureau’s College Park Metallurgy Research Center, College Park, Md., in lieu of a sample from their raw urban refuse processing pilot plant which at the time was not yet operating. One-fourth of the sample was characterized by hand sorting. Table 6 gives the sample composition as determined from the characterization study. The as-received sample was minus 3-inch pieces with a bulk density of 0.09 g/cu cm. The first step in separating the mixture into major types was chopping or shredding to minus 5/16-inch size in order to be compatible with our processing equipment. This operation increased the bulk density to 0.18 g/cu cm. The shredded material was subjected to air classification in the unit shown in figure 2. The air velocity was adjusted to remove most of the film and foam in the air-light fraction. The material was classified as 69 weight-percent air-light and 31 weight-percent air-heavy. Tests were conducted on the air-light fraction to separate the film and foam fractions by electrostatic separation. Samples containing 5 to 20 percent moisture in 5 percent increments were tested. The best separation was obtained at the 5-percent moisture level where the recoveries and grades for plastic film were 99 percent and 97 percent, respectively, and for foamed plastic 70 percent and 90 percent. These results are illustrated in graphic form in figure 16. The air-heavy material resulting from air classification was further separated into primary plastic types by hydraulic separation. In normal practice, using plastic derived from urban refuse, a cleaning step would be required prior to hydraulic separation. The results of hydraulic separation are shown in table 7. A sink-float analysis on each fraction was performed by static sink-floating each in water and calcium chloride solutions having densities of 1.20 g/cu cm and 1.50 g/cu cm, respectively. The overall process is shown in flow diagram in figure 17. Figures 18, 19, and 20 show the raw sample and the relative quantity of products derived from the application of the process in figure 17 to the synthetic waste plastics sample from College Park.

Wire Insulation Wastes
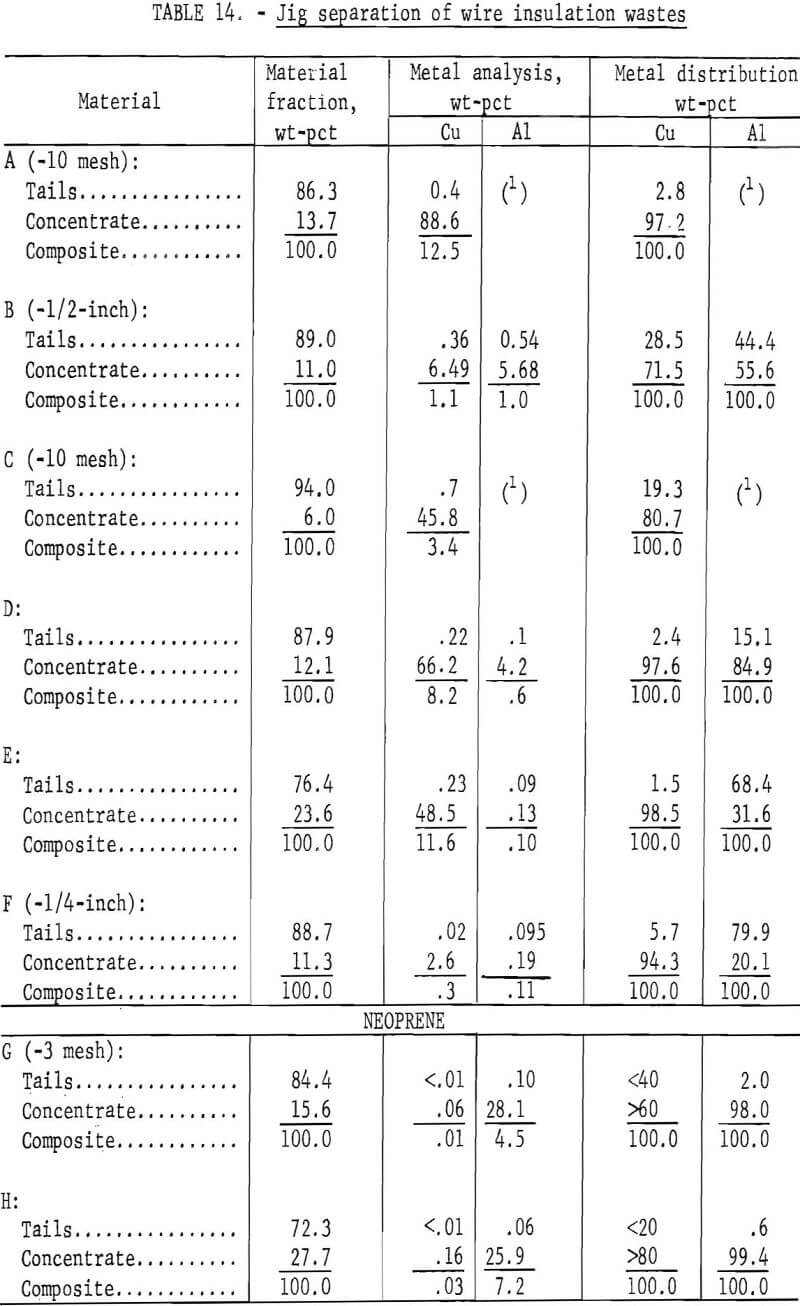
A large amount of copper and aluminum is recycled each year from scrap and reject wire. During the metal recovery process, a mixture of wire insulation choppings containing PVC, PE, neoprene, fiber, and 2 to 7 percent metal is generated as waste. Virtually no market exists for this waste material because of its mixed state and residual metal content. As a result, the most common disposal method for wire insulation waste is by landfill. Incineration is impractical because the major portion of the insulation is PVC from which hydrochloric acid is released during burning. An apparent solution to the problem would be to develop a process to remove the metal and segregate the PVC for recycling, leaving the balance for incineration.
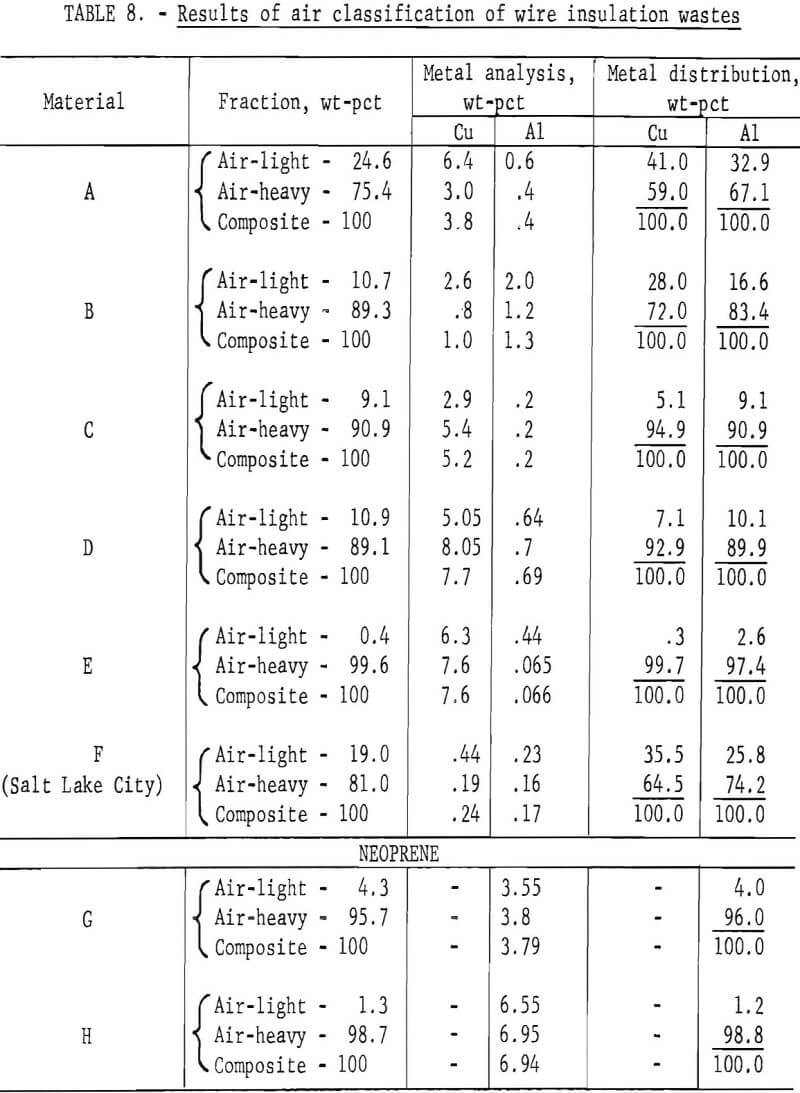
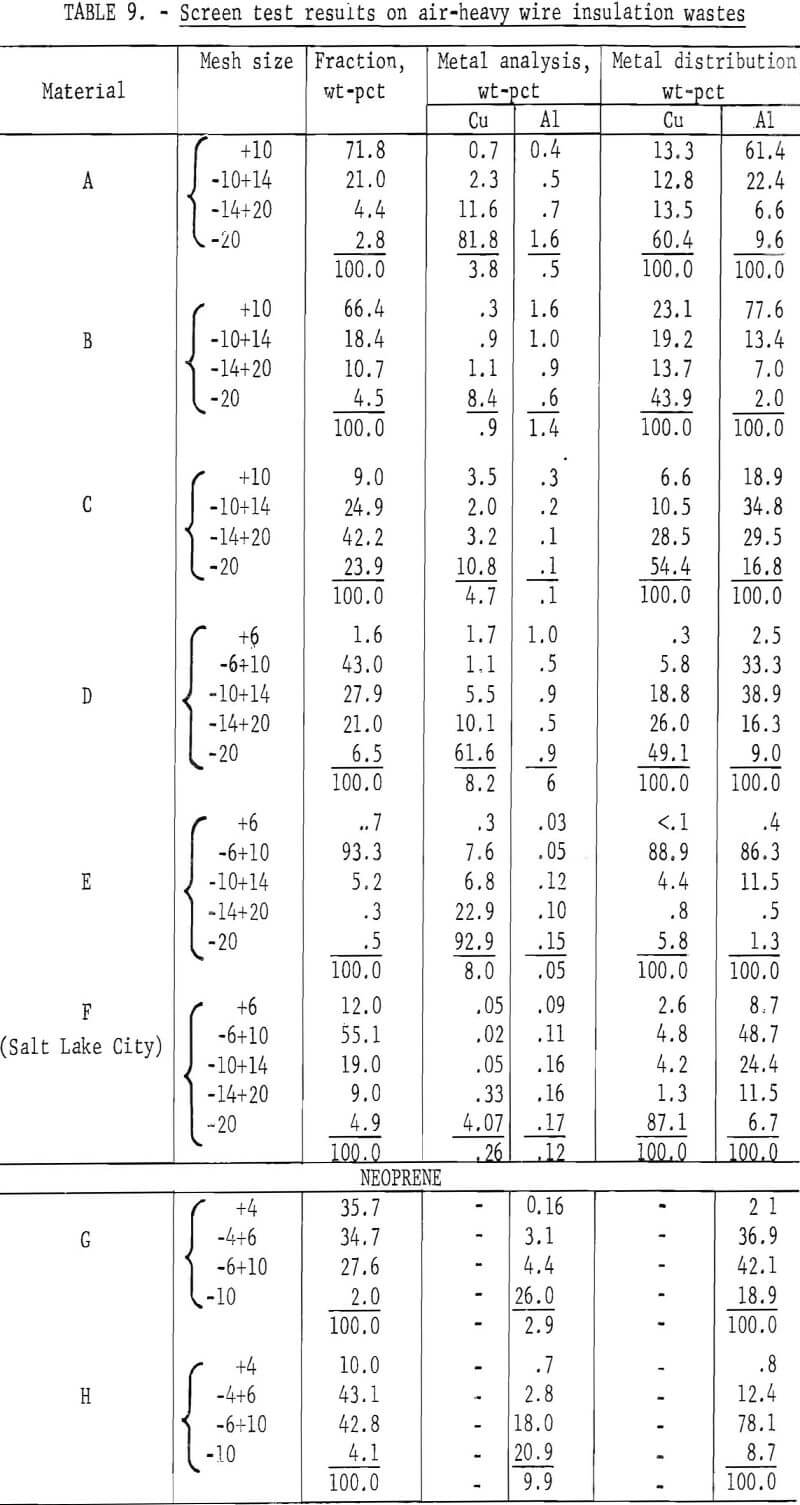
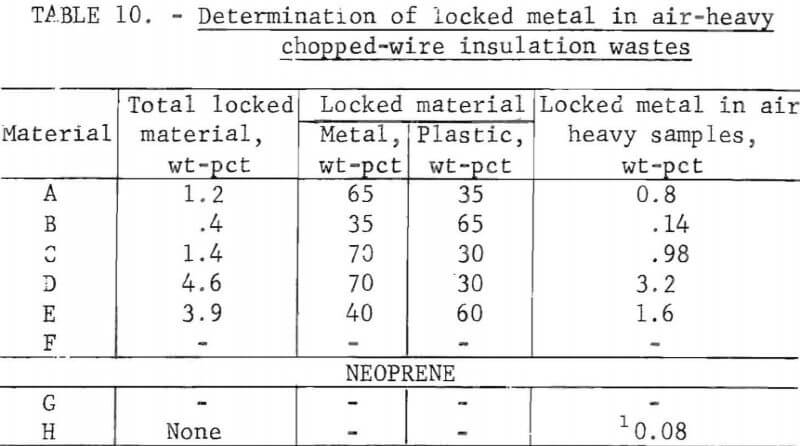
Wire insulation tailings samples from five commercial copper reclaiming processors and two aluminum reclaiming processors plus one sample from the Bureau’s Salt Lake City cryogenic separation process were characterized. This study determined metal content, size distribution, percent of locked metal, percent of air-light material, chlorine content, and density. The
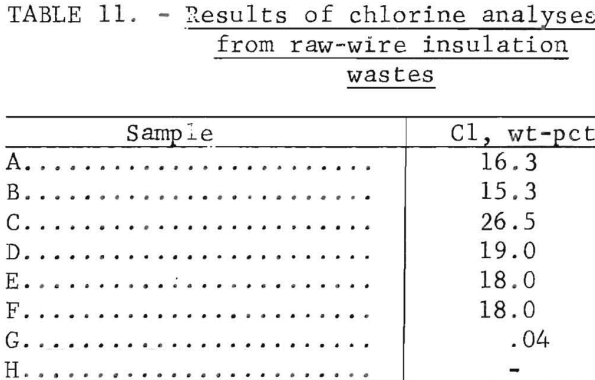
metal contents are shown both before and after air classification in table 8. In five of eight samples the air classified heavy material contained a higher percentage of metal than the raw sample. As can be seen from the table the raw samples contained from 0.4 to 8.4 percent metal, with the lowest residual metal occurring in the cryogenic sample from the Bureau of Mines Salt Lake City Metallurgy Research Center. Air classification removed a high of 24.6 percent air-light material to a low of 0.4 percent with the average percent air-light being 10 percent. Most of the tests were conducted on air-heavy fractions because of balling of the fibrous air-light material. Screen test results on air-heavy fractions from the same eight materials are shown in table 9. In most cases copper and aluminum were concentrated in the finer screen fractions. Locked metal, that is metal still attached to plastic, was determined by hand sorting two representative samples from air-heavy fractions of each waste. The results are reported in table 10. Most tailing samples contained about 1 percent metal in locked form. This type of determination was used to assess the efficiency of the metal recovery processing. If a sizeable percentage of metal is locked, chopping to a finer size will be necessary to improve the overall metal recovery. Chlorine analyses from raw tailings samples are presented in table 11. An indication of the amount of PVC resin in each waste can be estimated from this information. Most PVC base wire insulations contain about 50 percent PVC resin, 30 percent plasticizer, and 20 percent filler, stabilizer, lubricant, and coloring agents. Sink-float tests were conducted on samples from each waste. Separating media of water and two calcium chloride solutions with densities of 1.0 g/cu cm, 1.2 g/cu cm, and 1.5 g/cu cm, respectively, were used in the tests. Weight-percents in each density group are tabulated for air-heavy samples from each waste material and given in table 12. Additional information on the metal and chlorine content of some fractions is given in the same table. It can be seen from the chlorine analysis on materials A, B, and C that the highest chlorine content occurs in the material having densities between 1.2 and 1.5 g/cu cm. Also, the major portion of these materials is in this density range indicating a high percentage of PVC compound in the material. Using the previously mentioned information that PVC base insulation normally contains 50 percent PVC resin, the predicted chlorine content for 100 percent PVC compound would be 30 percent. Based on these assumptions, density fractions in the range of 1.2 to 1.5 g/cu cm of materials A and B contain 80 to 90 percent PVC compound. Obviously material C does not fit this pattern and must contain a higher percent of PVC resin than normal. As expected, most of the metal occurs in the >1.5 g/cu cm density fractions.
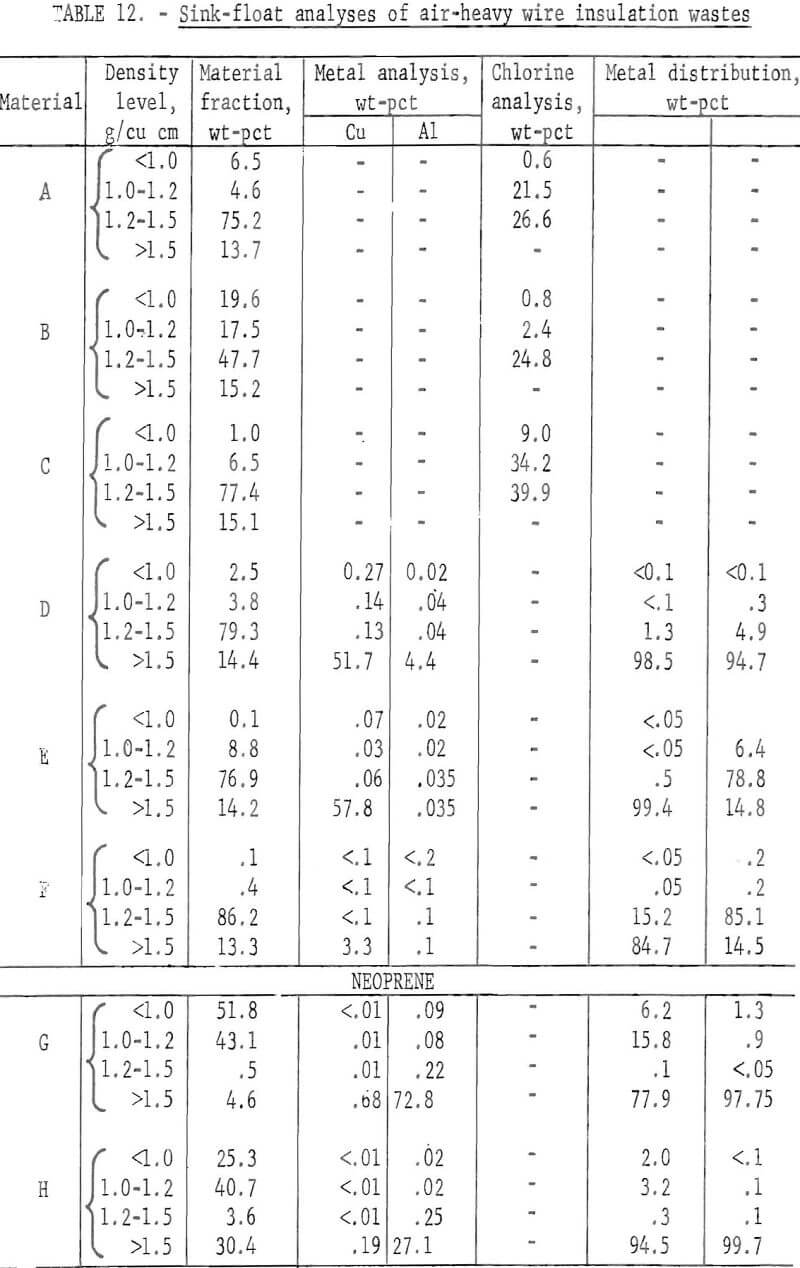
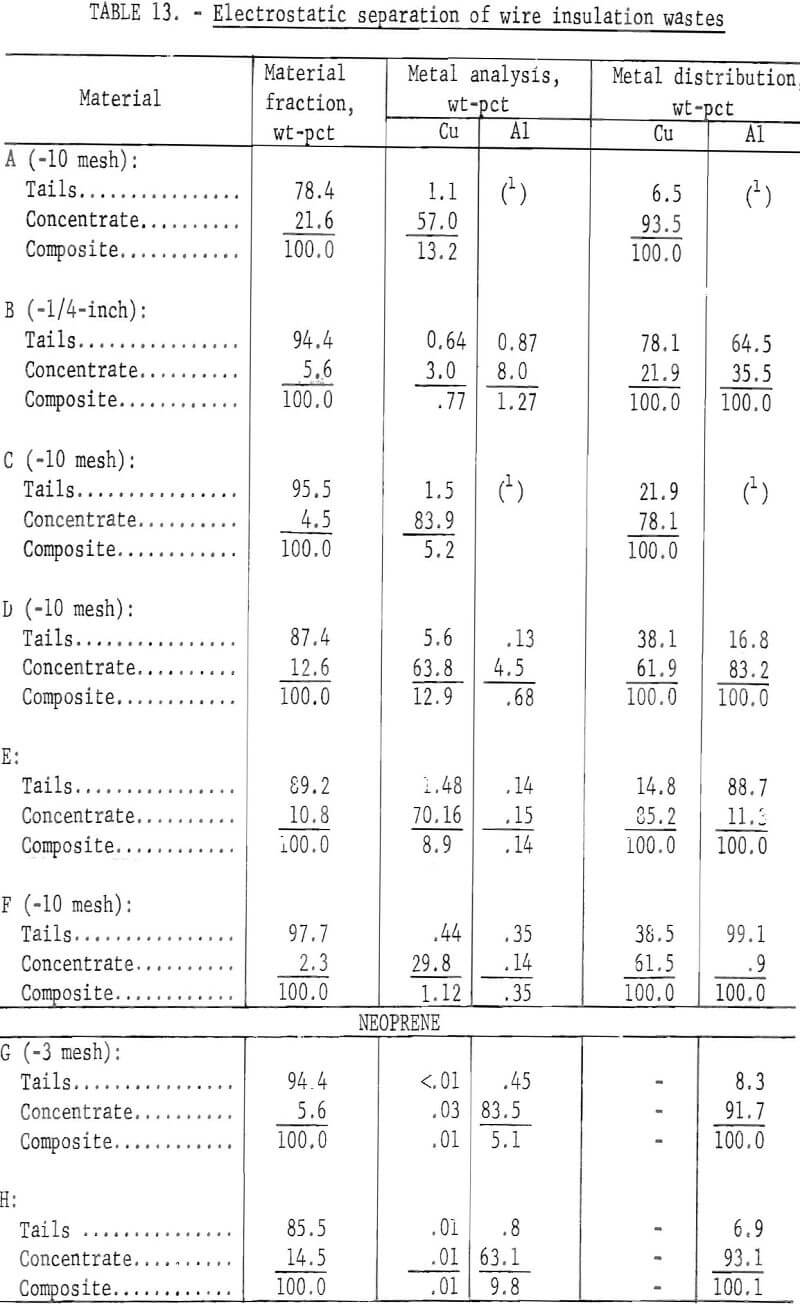
Two methods for lowering the residual metal content of these wastes were compared. These methods, electrostatic separation and jigging, were described in a previous section of this report. Air-heavy sized material was used in the tests. In some cases the material was screened prior to testing to upgrade the metal content. The results of both metal removal methods are shown in tables 13 and 14. The metal removal was better in all cases with the jig than with the electrostatic separator. In most materials the metal content was lowered to less than 0.5 percent. In the two neoprene samples the metal was lowered to 0.1 percent or less by jigging.
Potential Utilization Techniques
Refabrication
One method for reusing plastic waste, after separation into major types by the preceding operations, is the fabrication directly into new products by mechanical or other means. Mechanical methods for refabrication include compression molding, injection molding, and extruding. In the laboratory wafers 2 inches in diameter were easily made from chopped waste plastics in a laboratory press at pressures of 3,000 to 4,000 psig and temperatures of 150° to 170° C. Although the materials fused into impervious solids, the flow did not homogenize the plastics and the particles retained their bright colors. The method offers many ways of varying the ingredients of the mixture with virgin plastics, plasticizers, and inert filler and binder materials, Fabrication by hot pressing of traffic markers, parking barriers, paving blocks and tiles, and park benches and fixtures has been suggested as a useful way to utilize the waste plastics from urban refuse.
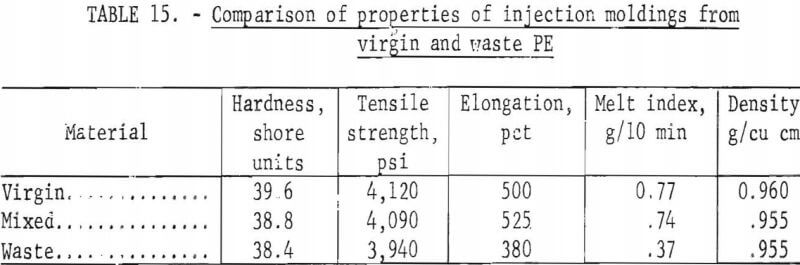
As described in a recent progress report, the injection molding experiments conducted with mixtures of waste PE, from bottles collected in the Rolla campaign, and virgin-injection molding grade PE were successful in producing fan fairings. However, fairings made from 100 percent scrap contained one small area where the mold did not fill. Melt index measurements made on the material indicated a melt index of less than 1, which is low for injection-molding-grade but normal for blow-molding-grade plastic from which bottles are fabricated. The addition of a small amount of high-melt-index material mixed with the waste to raise the overall melt index, causing improved flow, would undoubtedly have improved the filling of the mold. The results of physical and mechanical testing of specimens cut from the fairings of virgin, mixed virgin and waste, and all waste material yielded the information shown in table 15. Although more uniform material is required for injection molding than for hot pressing, it is a suitable high-volume method for fabricating products from waste plastics.
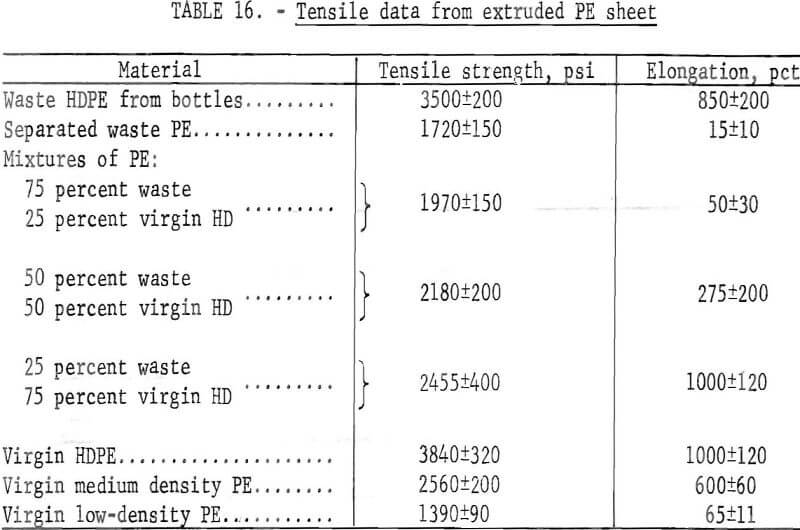
Laboratory experiments using a 1-inch extruder (figure 21) revealed that uniformity of melt index and a low level of contamination are more critical than for the other fabrication methods already mentioned. Extruded sheet 3-½ inches wide by 1/16 inch thick were made from three pelletized virgin PE materials and six granulated PE wastes and mixtures. Five tensile specimens were cut and tested from each of these extrusions and the average results are given in table 16. It is evident from the table that the tensile values for HDPE bottle waste are almost as high as those from virgin material. This is consistent with the fact that the waste bottle fraction represents a more uniform grade material than the separated waste PE which contains a mixture of high- and low-density PE having large differences in melt properties.
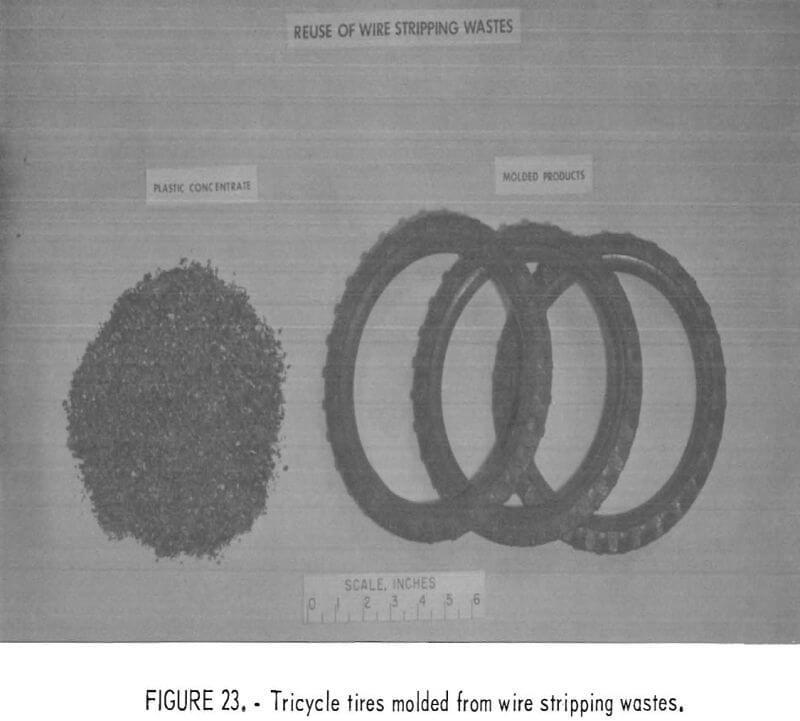
Mixed wire insulation wastes were fabricated into tricycle tires by intrusion molding (low-pressure injection molding) after the material had been air classified to remove fiber and dust and then jigged to lower the metal content to below 0.5 percent. These trial moldings were fabricated without additives. In practice, black coloring and plasticizer additives might be desired.
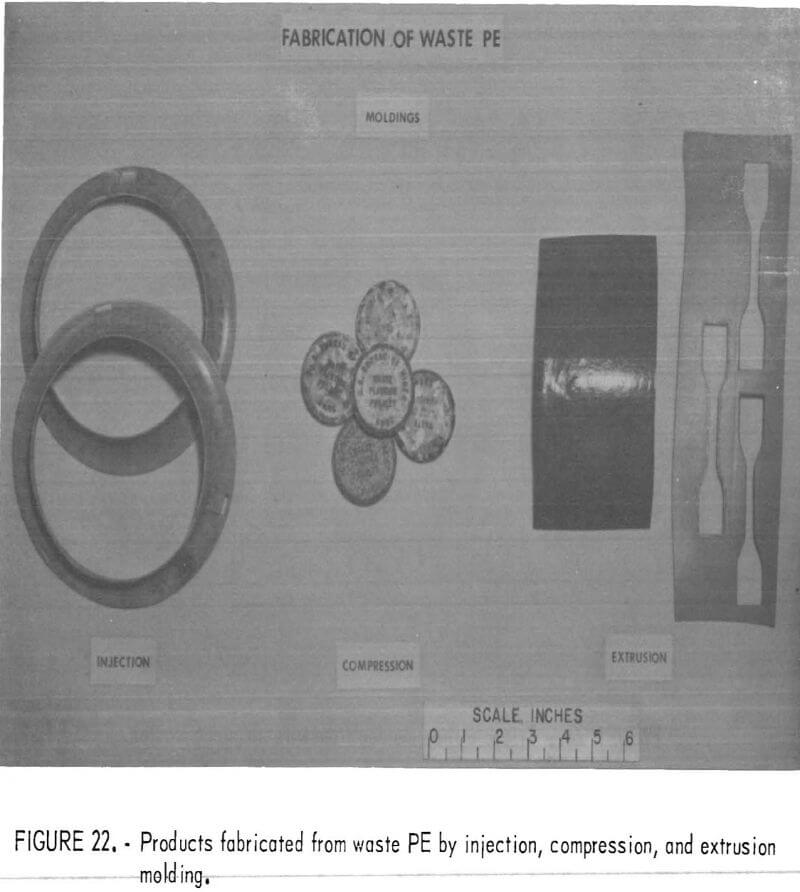
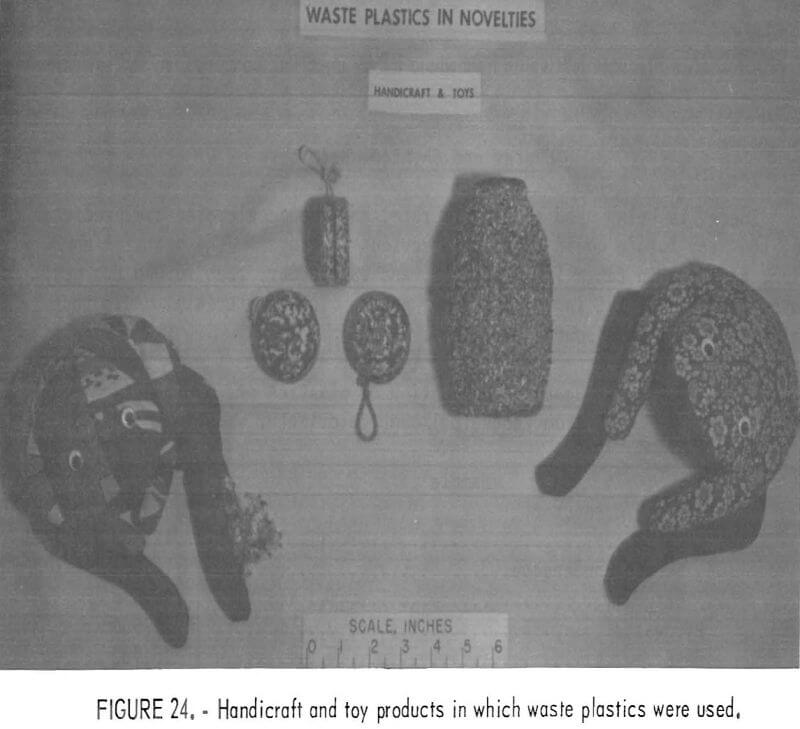
Numerous ways are available that require little or no machinery for reusing clean and chopped plastic waste. For example, a home craftsman, making children’s “bean bags” found cleaned, chopped, waste plastic to be an ideal sanitary, washable, and inert filler. Chopped waste plastic film, a major constituent in plastic from urban refuse, appears to have merit as a soil conditioner and plant starter. PVC wire insulation waste, mixed with plasticizer and cured, can be molded into a resilient pad which might be useful in sound and vibration control. Figures 22, 23, and 24 show some of the products possible from refabrication of waste plastics. Small industries may thrive on recycling of waste plastics if a consistent supply of resources flows from urban or regional waste processing plants. A recent study conducted for the Society of the Plastics Industry (SPI) indicates that there is much research and interest devoted to the recycling of waste plastics.

Energy and Chemical Recovery
Energy is being used at a rapid rate, and the accelerated use predicted for the near future could cause some serious shortages. The heats of combustion, as shown in table 17, of PE, PP, and PS are more than 16,000 Btu per pound and that of PVC is 9,900 Btu per pound. These materials, could thus be valuable as a future source of energy.
Pyrolysis, also known as destructive distillation, is a process where organic material is heated in a retort in the absence of oxygen or in a controlled oxygen environment. Investigations of pyrolysis by the Bureau of Mines and other organizations have led to the conclusion that this method of disposal of plastic wastes may have an economic advantage by generating more byproduct fuel gas than is needed to supply heat for the reaction. That other products from this reaction, such as organic liquids and char also have value as fuel, is expected since most plastics are derivatives of petroleum.
Pyrolysis then is a recycling method for plastics which will not only reduce the volume of waste, but from which valuable chemicals and fuel-grade products can be recovered. Polystyrene reportedly is one of the few polymers that yields a relatively high percentage of monomer when thermally decomposed.
In a previous paper, the production of hydrochloric acid and a char were advocated. It was also illustrated that, on the basis of freight charges in the southwestern United States, it costs twice as much to ship HCl in tank cars in the form of concentrated hydrochloric acid (20° Baume, 31.45 percent HCl) as it would to ship an equivalent amount in boxcars in combined form as polyvinyl chloride. Research to determine the feasibility and optimum parameters for the recovery of HCl from waste PVC was conducted. About half the weight (50 to 60 percent) of virgin PVC resin is HCl, but the addition of plasticizers and fillers dilute this amount. For example, two commercial blow-molding-grade PVC compounds contained 46 and 54 percent HCl. The PVC waste from the Rolla collection contained about 30 weight-percent HCl.
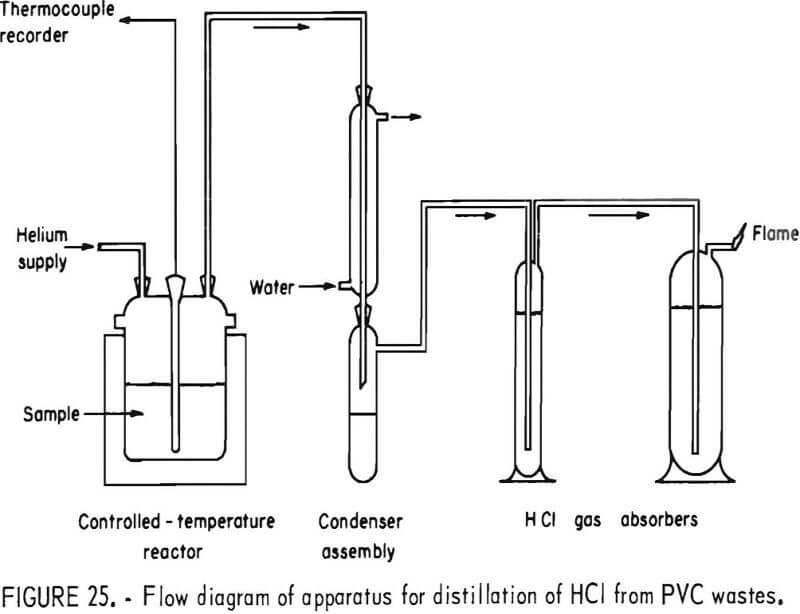
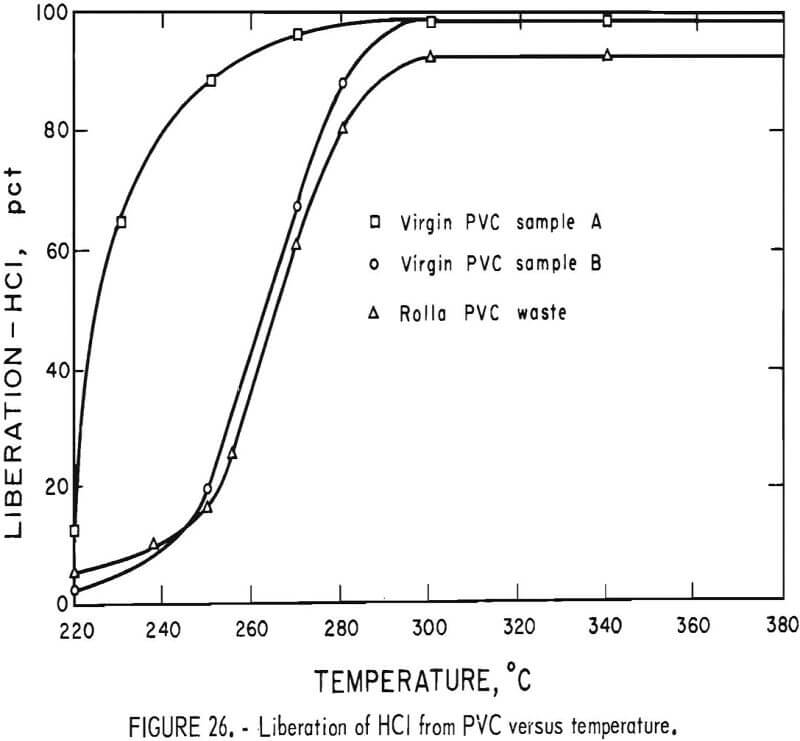
Thermal decomposition experiments of PVC conducted in a 1-liter reaction kettle using helium carrier gas showed that HCl evolution begins at 220° C and proceeds with increasing rapidity as the temperature is raised. A schematic diagram of the decomposition apparatus is shown in figure 25. Results of 1-hour thermal treatments of two virgin samples of PVC molding compounds and the Rolla waste PVC concentrate are shown graphically in figure 26. Dissociation was virtually complete after 1 hour at 300° C, liberating 99 percent of the HCl in the virgin samples and 92 percent in the waste. The other products of the dissociation, the char, the liquid condensate, and the offgas all have fuel value. Isothermal bomb calorimeter measurements were made on these products and on the major types of thermoplastics the results of which are shown in table 17. The char from the waste PVC was 44 percent of the original sample and contained 6.3 percent water, 15 percent ash, 51 percent volatile matter, and 27 percent fixed carbon. From 10 to 15 percent of the PVC from the Rolla collection dissociated as liquid condensate, which contained benzene, toluene, xylenes, and a heavy oil, and when burned left 1 percent ash. The offgas, 10 to 19 percent of the waste PVC, was shown by mass spectral analyses to contain water, methane, ethane, propane, and butane. The optimum decomposition temperature for waste PVC was determined to be 350° C. Most of the unrecovered HCl was found in the char, having reacted with the calcium carbonate filler material to form calcium chloride. Compounding PVC with basic fillers, such as calcium carbonate, potassium carbonate, magnesium oxide, and others, reduces the outflow of HCl during pyrolysis. Water leaching of the char provided a 98-percent removal of HCl from the PVC waste.
Thermal decomposition of waste-wire insulation tailings at 300° C for 1 hour resulted in 72 to 93 percent liberation of HCl. The percentages of char, liquid condensate, and offgas were very similar to those from the waste PVC tests. It was noted that liberated HCl reacted with the residual aluminum and copper metal to form metal chlorides which remained in the char fraction. Water leaching of the char lowered the residual chlorine in the char to 1 percent, thus providing comparable HCl recovery to that from the PVC waste. Tests at 400° C showed very little increase in HCl liberation, but the liquid condensate increased from 6 to 10 percent. At the higher temperature a smaller amount of char and an increased amount of offgas was obtained. Ash from the char contained minor amounts of Al, Fe, Cu, Mg, Pb, Sn, and Ti and preponderant amounts of Ca and Si.
It is evident that waste PVC merits consideration in chemical processing as a resource for HCl and fuel. Waste PVC could offer advantages, for the consumer in outlying regions, owing to its flexibility in supply, storage, and use; to the low hazard in its shipping and storage; and to low shipment costs.
Conclusions
During this era of environmental concern, the plastics industry is being faced with numerous problems ranging from legislative proposals requiring recycling of post-consumer products to material shortages owing to the energy crisis. Since the petroleum resources from which most plastics are derived are a fixed quantity, it behooves the plastics industry to use its share of this resource judiciously. These Bureau of Mines laboratory experiments show that numerous optional courses exist for processing, recycling, and utilizing plastics from urban and industrial wastes. One of the major contributions made by this research was in the area of separation technology. It has been demonstrated that thermoplastics can be segregated from other materials into primary types based on variations in density, size, and electrical properties. Both wet and dry separation methods were shown to be effective. Although the purity of secondary plastics is generally lower than that of virgin materials, utilization of these materials in areas of refabrication, chemical recovery processes (pyrolysis), and for fuel could be considered alternative uses that would be more beneficial than disposal. From which of these options the maximum benefit could be derived is a question that depends on a number of factors, and one that solid waste managers will have to answer in the near future as large urban refuse processing systems begin to search for secondary material markets. It is hoped that some of the results of the research and development described in this paper will serve as guidelines in the design or selection of processes to recover and reuse waste plastics from urban waste treatment plants.
