Table of Contents
Chromite ore in combination with various quantities of magnesia is used in basic refractories. Refractories of this type are used as linings in steelmaking and copper smelting furnaces, rotary cement calciners, and glassmaking tanks. However, most of the consumption is by the copper and steel industry. Approximately 20 percent of the chromite consumed in the United States is used for refractory purposes.
As part of our objective to maximize minerals recovery from secondary domestic resources, we investigated techniques for reclaiming, reforming, and reusing chromite containing waste refractories from steelmaking.
Description of Samples
Samples of waste magnesia-chrome refractories from argon-oxygen decarburization (AOD) and electric steelmaking operations were obtained from two steel producers for this study. The samples were from plants in Ohio and Pennsylvania. The companies did not provide estimates of the quantity of materials generated or stockpiled. However, in 1974, the steel industry consumed 4.9 pounds of Cr2O3 as chrome-bearing refractories per ton of steel produced.
The samples of waste refractories consisted of whole and parts of used refractory brick. Some of these brick were partially enclosed in metal casings, others had metal and slag readily visible in cracks, and some were contaminant-free.
Chemical Analyses
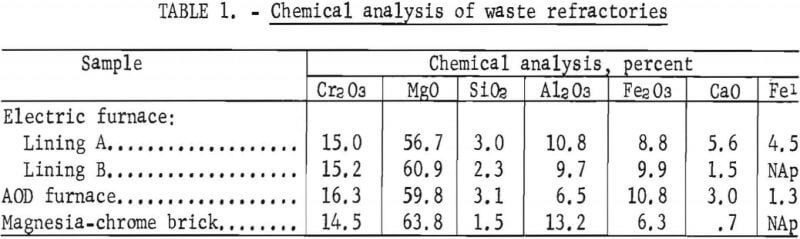
Chemical analyses were made of the head samples from each waste refractory to quantify the refractory constituents and contaminants. The head samples had been handpicked to recover the metal casings and magnetically separated to recover large pieces of magnetic slag and iron contaminants. Chemical analyses were also made of a commercial refractory brick for comparative purposes and to serve as a standard for beneficiation. These analyses (table 1) indicate that the waste refractories were of similar composition to the unused commercial brick.
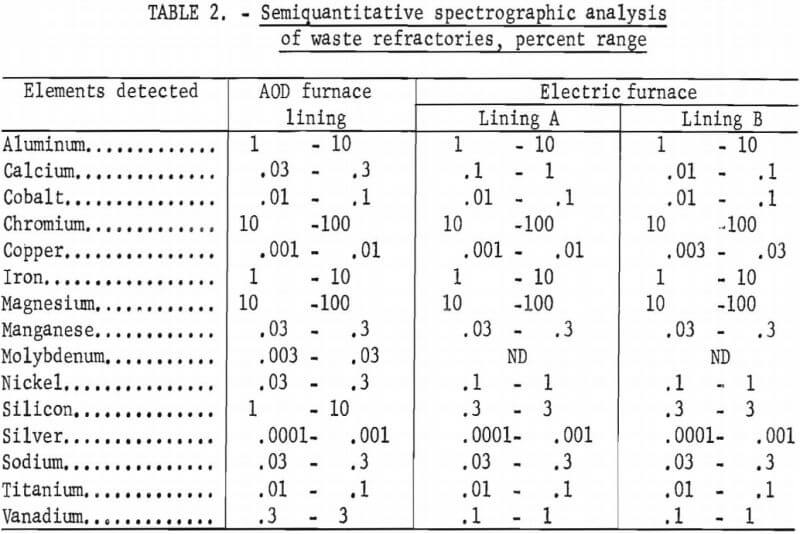
Energy dispersive X-ray analysis showed only Mg, Cr, Al, Fe, Si, and Ca as the major elemental constituents of the samples. However, traces of Mo, S, V, and Ti were also observed in the AOD furnace sample, and Ti and Zn were observed in the electric furnace samples, Semiquantitative spectrographic analyses, (table 2) indicated that trace amounts of Ag, Co, and Ni were also present.
Mineralogical Analyses
X-ray diffraction, energy dispersive X-ray, and semiquantitative spectro-graphic analyses were made of each sample for mineralogical determinations and trace element identification. X-ray diffraction patterns were also valuable in determining that no significant phase changes occurred in the refractory grain during furnace operations that might alter the refractory properties of the beneficiated refractory grain (chrome concentrate).
Chromite ((Fe,Mg)O·(Cr,Al,Fe)2O3), periclase (MgO), and minor amounts of forsterite (Mg2SiO4) were identified in all the samples.
Particle Size Distribution
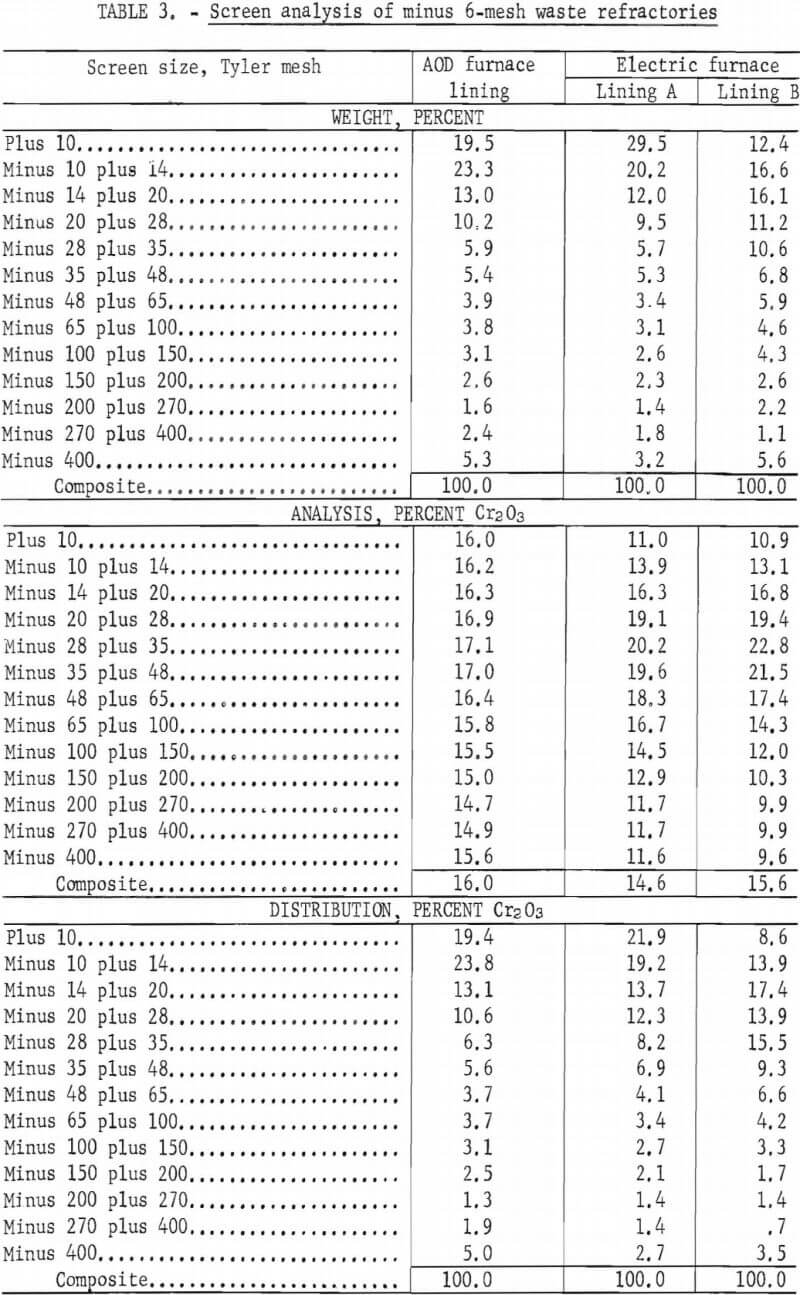
Chemical analyses were made of the various size fractions of each sample of the refractory wastes to determine the percent of Cr2O3 in each fraction. The size analyses, Cr2O3 content, and Cr2O3 distribution in the various size fractions are shown in table 3.
While there is some variation in the Cr2O3 content of the various size fractions, none are low enough in grade to be rejected as a tailings product. The material was crushed to 6 mesh prior to examination because this is the size range most amenable to the manufacture of refractory brick. Microscopic examinations of the size fractions from the screen analyses were made to ascertain the particle size at which contaminants were liberated from the refractory grain. Most of the contaminants were observed to be liberated at minus 6 mesh.
Beneficiation Studies
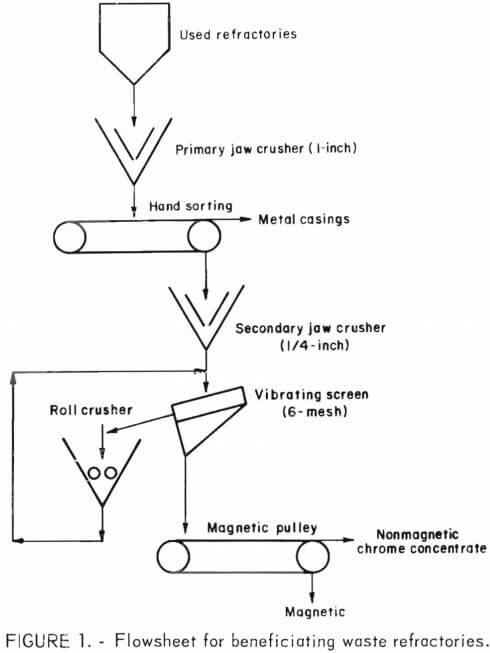
The waste refractories were crushed, screened, and magnetically separated as presented in the flowsheet of figure 1. The metal casings from handpicking and the magnetic product from the magnetic pulley were combined. This combined product accounted for 1.3 and 4.5 percent of the total weight received for the AOD and electric furnace waste refractories, respectively.
After removing the metal casings and magnetic contaminant, chemical (table 1) and X-ray analyses made of the nonmagnetic product or chrome concentrate were comparable to those of the commercial brick.
To determine if a magnetic separator could remove an appreciable magnetic contaminant fraction at various size of grinds, several magnetic separations were made. Waste refractory samples were crushed to pass 6, 10, 20, 35, and 65 mesh and were dry magnetically separated using a Carpco low-intensity rotating-field magnetic separator at its maximum field intensity. Other waste refractory samples were crushed to pass 6, 14, 28, 48, and 100 mesh and were wet magnetically separated using a Davis tube magnetic separator operated at its maximum magnetic field intensity.
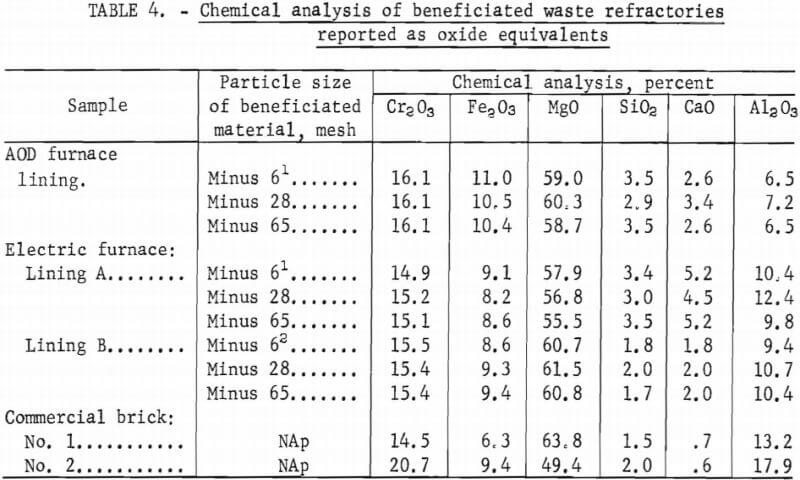
The small quantities, 0.1 to 3.0 percent, of magnetic fraction were analyzed for iron, and complete analyses were made for the nonmagnetic refractory fraction. Chemical analyses of the minus 6, 28, and 65 mesh nonmagnetic refractory fraction are shown in table 4. Neither finer crushing nor the use of the high-intensity magnetic separator would effect a separation of an appreciable quantity of magnetic impurities.
After comparing chemical analyses of the beneficiated refractory wastes to the commercial refractory, minor differences in silica (SiO2), alumina (Al2O3), and calcium (CaO) were observed. The differences were so small that no further beneficiation efforts were made.
Refractory Evaluation
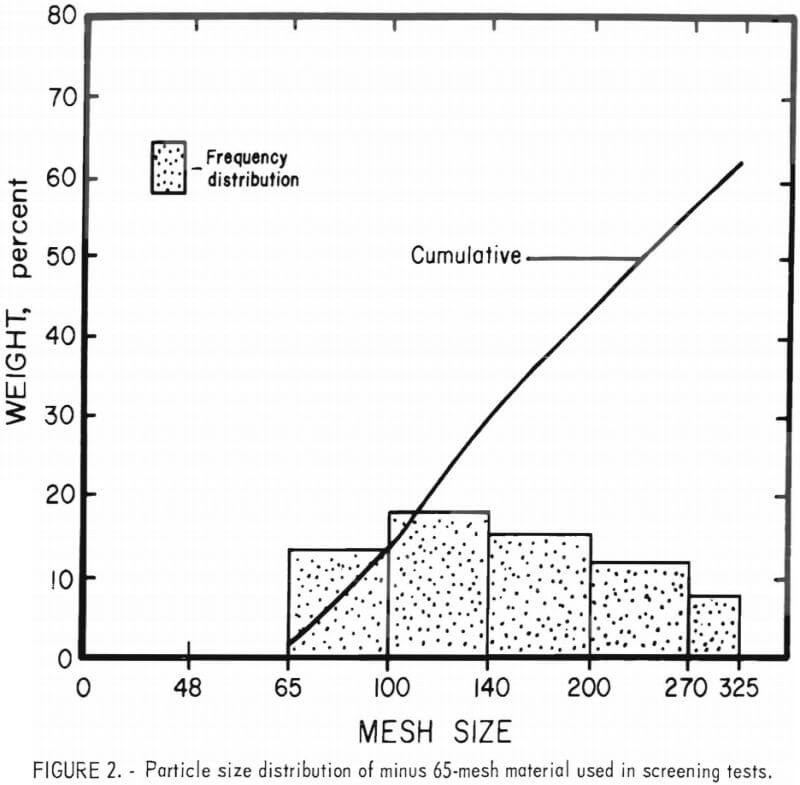
Preliminary investigation indicated that the particle size distribution of the chrome concentrates significantly affected the refractory properties of sintered samples. To eliminate this parameter from the results, all samples, from both the beneficiated and “standard” materials, were carefully stage- ground by hand to yield the distribution shown in figure 2. It should be noted that this minus 65 mesh distribution is not typical of the distribution used in the production of full-size refractory brick, but was required owing to the small sample size used in this evaluation.
The beneficiated chrome concentrates from each refractory waste (two electric and one AOD) ground to minus 65 mesh were mixed with 3 percent poly-ethylene glycol, pressed into 1- by 2- by ¼-inch-thick briquets at 20,000 psi, and sintered at 1,500° C in a gas-fired furnace. The briquets were then sectioned into ¼- by ¼- by 2-inch-long bars for modulus of rupture, density, and porosity determinations. Chemical, X-ray diffraction, scanning electron microscope, and energy dispersive X-ray analyses were also used to study changes in mineralogy and intergrain bonding.
Commercial refractory bricks of identical or similar chemical composition were crushed to the same particle size distribution as the beneficiated material, and following the procedure described above, samples were prepared and used as standards for comparison.
The chemical analyses of the three beneficiated steel plant refractories along with analyses of two commercial bricks are presented in table 4. Both the SiO2 and CaO contents were slightly higher in the beneficiated materials. No “free” lime, present as CaO, was detected by chemical analysis in any of the samples. In commercial magnesia-chrome refractories, the Cr2O3, Fe2O3, MgO, and Al2O3 contents vary over wide limits depending on the source of the chrome ore and the chrome-periclase ratio. The variation of these oxides in the materials tested falls well within these ranges.
Table 5 shows the 1,350° C modulus of rupture, bulk density, and apparent porosity values for samples prepared from chrome concentrates beneficiated at minus 6, minus 28, and minus 65 mesh for each of the three starting waste materials, along with values for samples prepared from two commercial bricks. Similar values were obtained from samples prepared from material beneficiated at minus 10, minus 14, minus 20, minus 35, minus 48, and minus 100 mesh, indicating that contaminants were sufficiently removed through beneficiation of a minus 6-mesh material.
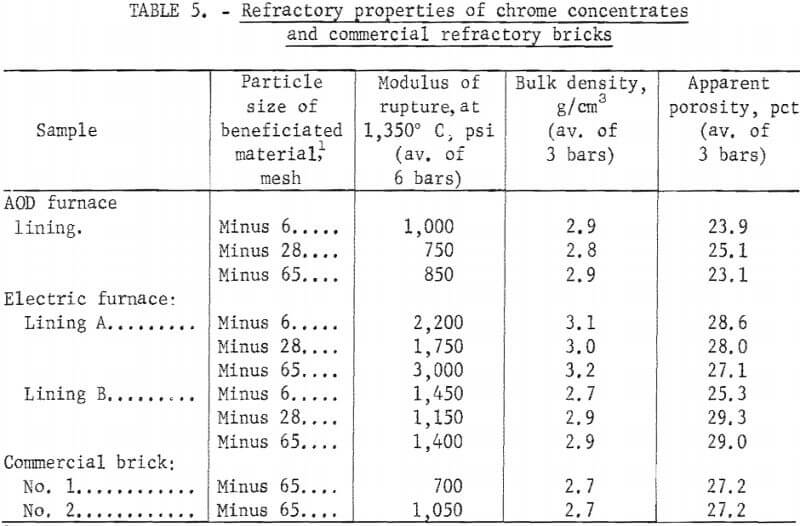
Owing to the small particle size of the material and the relatively low firing temperature (1,500° C), the values reported in table 5 should be interpreted only as a means of comparing the test materials to the commercial refractories and not as absolute. These results do indicate, however, that refractories produced from the beneficiated materials compare favorably to similar test specimens prepared from commercial refractory grain using the same procedure.
X-ray diffraction analysis showed that all samples contained primarily chrome spinels, periclase, and forsterite, with trace amounts of monticellite, alpha-iron, and magnetite, after firing to 1,500° C. With the firing temperature increased to 1,700° C, only chrome spinels, periclase, and forsterite were detected.
A scanning electron micrograph and elemental scans of Cr, Mg, Fe, and Al of a typical sample fired to 1,500° C are shown in figure 3. As can be seen, following firing at 1,500° C, the sample contains an intimate mixture of periclase and chromite grains with the presence of a small amount of intermediate glassy-bond phase. When samples were fired to 1,700° C, direct bonding between periclase and chromite increased with a substantial decrease
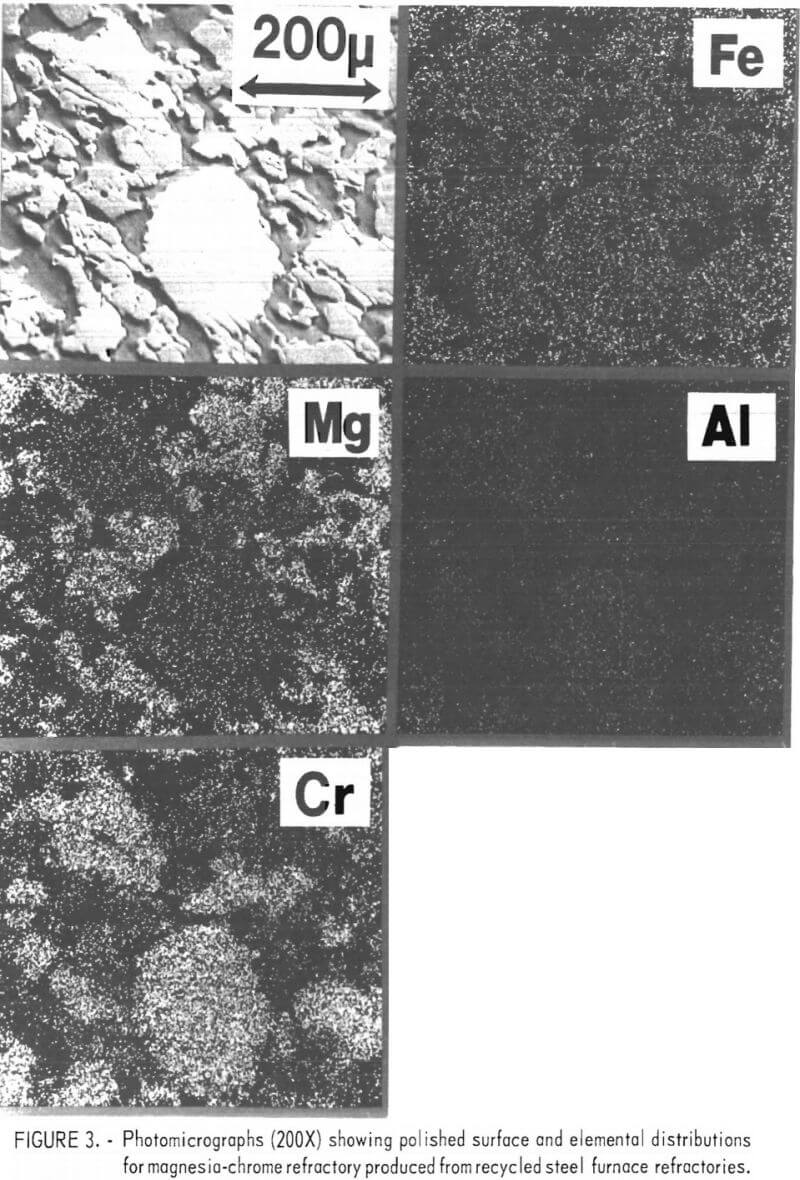
in the amount of glassy phase present at the grain boundaries. This results from a redistribution of the silica from reaction with MgO to form forsterite and with Ca+2 to form dicalcium silicate, both having high melting points and good refractory behavior.
Environmental Considerations
An environmental study was made as a part of the recycling process for the refractory wastes. This study included identifying compounds, elements, or ions that might have adverse effects on the environment as a result of recycling efforts. Characterization and a literature survey suggested the possible presence of hexavalent chromium (Cr+6) in varying quantities. The threshold limit values (TLV) for hexavalent chromium in workroom air have been set at 0.5 milligram per cubic meter.
Quantitative determinations were made for hexavalent chromium on a new magnesia-chrome refractory brick, a sample of Montana chromite, and head samples of the waste refractories. The analyses were made by the analytical staffs of the Albany, Rolla, and Tuscaloosa Research Centers. Results are presented in table 6. Although the same analytical procedure was followed by each group performing the analysis, there was a wide variation in the reported analyses. However, some of the data show close correlation.
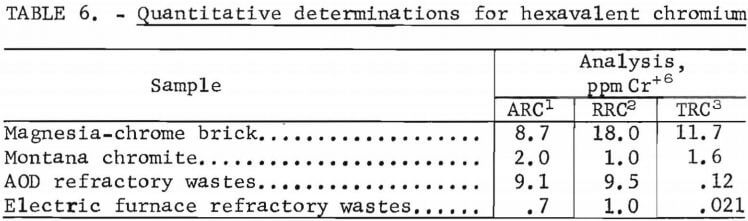
The recycling process for the waste refractories is a dry process. The precautions to prevent overexposure would include using a good ventilation system and ensuring that employees wore approved respirators. Calculations based on 20 ppm Cr+6 in the dust (from table 6) and a TLV of 0.5 mg Cr+6/m³ show a TLV for dust of 25 g/m³.
During crushing of a 10-ton sample of waste refractories, officials from the U. S. Department of Labor, Mining Safety and Health Administration (MSHA) monitored dust levels and employee exposure by sampling the crushing area. The time-weighed exposures did not exceed the TLV in any of the samples collected.
Summary and Conclusions
By using a combination of crushing, screening, and dry magnetic separation, it was possible to beneficiate waste AOD and electric steel plant refractories using conventional beneficiation techniques. It was found that contaminants were liberated by crushing the waste refractories to minus 6 mesh.
Based on 1,350° C modulus of rupture, density, and porosity values, samples produced from beneficiated materials compared favorably with samples prepared from commercial brick of similar composition. By producing recycled brick from material beneficiated at minus 6 mesh, only minor adjustments to the particle size distribution would be required to produce full-size brick by conventional techniques.
In Bureau of Mines research on recycling chrome refractory wastes, used refractories from argon-oxygen decarburization and electric steelmaking furnaces were beneficiated, concentrates were reformed into briquets, and modulus of rupture tests were conducted at 1,350° C. The test results indicated that the beneficiated minus 6-mesh material could be used for producing magnesia-chrome refractories.