Table of Contents
- Materials
- Nickel Recovery by Carbonyl Formation
- High-Temperature Chlorination
- Oxygen Pressure Leaching
- Ammonium Salt-Oxygen Leaching
- Acid-Oxygen Leaching
- Chlorine-Oxygen Leaching
- Flowmeter-Gas Chromatography
- Atomized Scrap Leaching
- Aluminum-Chromium-Iron Precipitation From HCl-O2 Pregnant Solution
- Room-Temperature Hydroxide Precipitation
- Scavenger Metal-Oxygen Treatment
- Hydrothermal Precipitation
- Comparison of Scavenger Metal-Oxygen Treatment and Hydrothermal Precipitation
- Solution Purification
- Caustic Fusion of Oxygen Pressure-Leached Residue
- Chromium-Iron Reduction
- Proposed Leaching-Purification-Electrowinning Procedure
We treated mixed and contaminated superalloy scrap by pyrometallurgical and hydrometallurgical methods to separate and recover metal values. Best results were obtained by leaching Zn-treated or atomized scrap with HCl-O2 at 95° C and 50 psig O2. This resulted in dissolving approximately 98% of the Al, Co, Cr, Cu, Fe, Mn, Ni, and Zn while rejecting over 98% of the Mo, Nb, Ta, Ti, W, and Zr as an insoluble refractory residue. Chlorine was successfully substituted for HCl to leach Zn-treated scrap but was unsuccessful for leaching atomized scrap. The leaching solution was treated by pH adjustment and hydrothermal precipitation at 200° C for 4 h to remove Al, Cr, Fe, and other contaminants as a filterable precipitate. Recovery of Co and Ni would be accomplished by solvent extraction and electrowinning. Chromium recovery as a ferroalloy was demonstrated.
The Bureau of Mines is investigating techniques to recycle contaminated bulk superalloy scrap by sequential pyrometallurgical and hydrometallurgical methods. Cobalt- and nickel-based superalloys have critical applications in the aerospace and power-generation industries; they are used in turbines and other high-temperature equipment that require oxidation resistance and high strength at elevated temperatures. The United States must import a large percentage of the metals that are used in superalloy production. Examples of such metals and the U.S. net import reliance for each metal in 1987 follow, in percent: Nb(Cb), 100; Ta, 92; Co, 86; W, 80; Cr, 75; and Ni, 74;
A Bureau study disclosed that 82,000 st of contaminated superalloy and similar high-temperature, corrosion- resistant scrap was downgraded, lost, or exported in 1976. This scrap is a possible source of up to 32,000 st Ni, 15,000 st Cr, 4,000 st Co, and 31,000 st of the other alloying metals.
The physical and chemical properties that make superalloys useful also make them difficult to treat for recovery of their constituent metals. Unless the alloys are kept separated during fabrication or the exact scrap composition is known, the scrap cannot be directly remelted for use in jet engines or other critical applications. If there is doubt about the alloy type or if different alloys have been mixed, the scrap must be downgraded or exported. The research reported here is concerned with mixed, contaminated superalloy scrap of this type.
Researchers have investigated a number of pyrometallurgical and hydrometallurgical methods to separate and recover the constituent metals from superalloy scrap. Brooks used chlorinated HCl to dissolve superalloy scrap, followed by solvent extraction and precipitation to recover the constituent metals as oxides, carbonates, and basic sulfates. Kenworthy, DeBarbadillo, and Kusik also investigated the recovery of metals from superalloy scrap but treated only one particular alloy or recovered one particular metal. A single method capable of treating all types of superalloy scrap to recover a number of different metals would be advantageous.
This investigation studied pyrometallurgical and hydrometallurgical methods to separate and recover the constituent metals in mixed, contaminated superalloy scrap to recover metals of sufficient purity to be used in the production of turbine- or jet-engine-quality superalloys. The primary concern was to maximize recovery of Co and Ni. Techniques investigated included Ni recovery by carbonyl formation, high-temperature chlorination, O2 pressure leaching, selective precipitation of contaminants present in O2 pressure-leaching solutions, caustic fusion of O2 pressure-leached residue, and pyrometallurgical reduction of Cr and Fe.
Materials
The superalloys investigated were representative of Co- and Ni-based superalloys. The particular alloys were cast Ni-based IN-738 and B-1900+Hf, cast Co-based Mar- M-509, and bulk contaminated scrap acquired from a commercial superalloy remelter. A 40-lb (18.16-kg) sample of each of the four types of scrap was melted with 160 lb (72.64 kg) of Zn at 850° C, and the Zn was removed by vacuum distillation at 750° C. The treatment transformed the superalloy into a metallic sponge that was friable, had a surface area of about 0.5 m²/g, and readily dissolved in acid. Analyses of the four types of scrap after Zn treatment are listed in table 1.
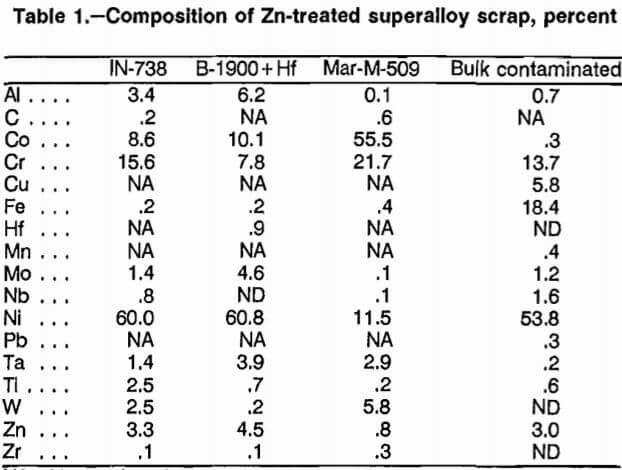
A scrap composition representing a weighted average for superalloy production in 1976 was calculated from data in a Bureau publication. The calculated average is listed in table 2. A mixture was prepared with Zn-treated IN-738, Mar-M-509, and the bulk contaminated scrap to approximate the calculated industrial average scrap composition. The composition of this simulated industrial average mixture is listed in table 2. Experiments used the four types of Zn-treated superalloy scrap described in table 1 and the simulated industrial average mixture. (The phrase “Zn-treated superalloy” will be dropped for brevity, and further references to scrap in this paper will refer to the four types of Zn-treated scrap and the simulated industrial average mixture.)
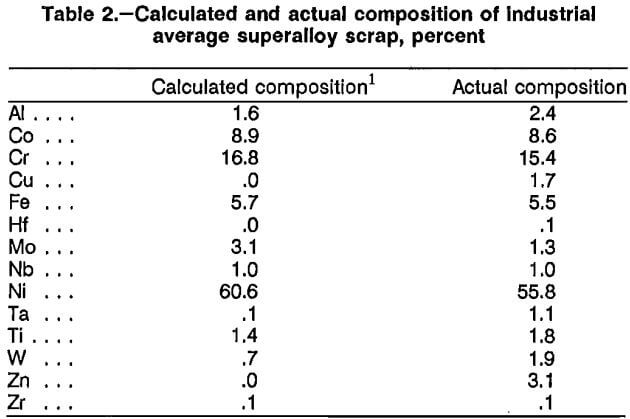
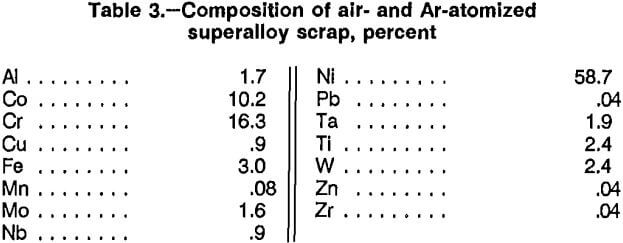
Atomization of molten superalloy scrap to produce a finely divided metal powder was a second technique employed to increase surface area. A private company was contracted to convert a sample of superalloy scrap into a metal powder using its proprietary atomization process. The scrap sample had not been previously Zn-treated. Atomization was performed both in air and Ar atmospheres after the metal had been melted at 1,700° C. The chemical analyses of the air- and Ar-atomized scrap were identical and are listed in table 3. Oxygen contents of the two atomized scraps were determined. The air-atomized scrap contained 0.046 pct O2, and the Ar-atomized scrap contained 0.015 pct O2. The atomized scrap had a surface area of 0.04 m²/g, and 48 pct of the material was minus 400 mesh.
All chemicals used during the project were of reagent grade.
Nickel Recovery by Carbonyl Formation
Carbonyl experiments used IN-738 and Mar-M-509 scrap. The scrap was treated with high-pressure carbon monoxide (CO) at elevated temperatures to form volatile nickel carbonyl, which could be removed and decomposed to metallic Ni. CO was introduced at 10 atm into a vertical stainless steel reaction tube containing the sample supported on glass wool and heated to 100° C by a resistance-heated tube furnace. The exit gas was expanded to atmospheric pressure and allowed to flow through a glass tube heated to 400° C, where the nickel carbonyl was decomposed to Ni metal and CO. Experiments were conducted on as-treated scrap and on scrap that had been pretreated by heating to 400° C for 2 h in a flowing H2 atmosphere or leached in 6N NaOH (sodium hydroxide) for 3 h at 95° C.
Treatment of scrap with CO resulted in conversion of 6 pct of the Ni, which was recovered as metal. There was no appreciable increase in Ni recovery for scrap pretreated with H2. Pretreatment of scrap by leaching with NaOH resulted in conversion and recovery of 12 pct of the Ni. There was no difference in results between the IN-738 and Mar-M-509 scrap. Research on this method was discontinued because of poor Ni recovery.
High-Temperature Chlorination
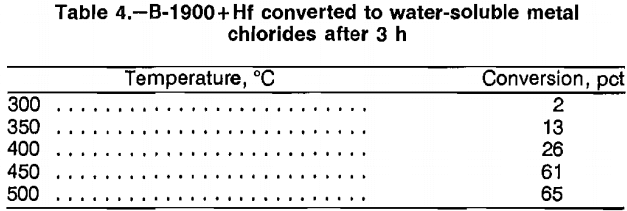
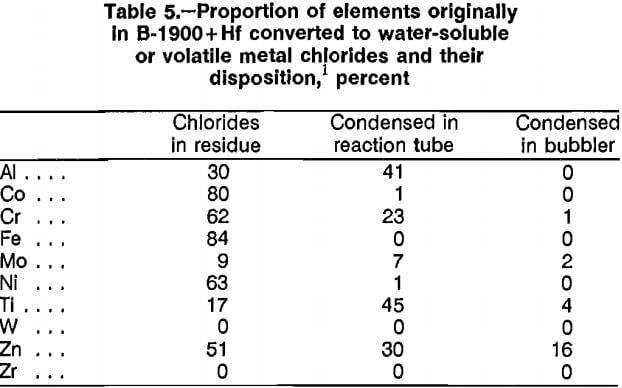
Scrap was treated with Cl2 gas at atmospheric pressure and elevated temperature to form volatile or water-soluble metal chlorides. Chlorine gas was introduced at 300 cm³/min into a vertical Pyrex heat-resistant glass reaction tube containing 2.5 g of scrap and heated by an electric resistance tube furnace. The gas exited through a bubbler filled with water. Temperatures investigated ranged from 300° to 500° C. Reaction time was 3 h. A portion of the volatile metal chlorides condensed at the cool end of the reaction tube, and the remainder was trapped in the water bubbler. The residue was leached with water to dissolve the soluble metal chlorides. Results from experiments performed on B-1900 + Hf scrap (table 4) show that increased temperature increased metal conversion. Table 5 describes the disposition of the
constituent metals in the experimental apparatus and gives the percent conversion of elements originally in scrap to soluble or volatile chlorides after treatment at 500° C for 3 h. The balance of the material remained unreacted in the residue. Investigation of this technique was discontinued because of lack of selectivity and incompleteness in the conversion of metals to soluble chlorides.
Oxygen Pressure Leaching
Investigation of O2 pressure leaching of scrap was performed with three reagent systems: (1) ammonium salts, (2) sulfuric acid (H2SO4), and (3) hydrochloric acid (HCl). The choice of these systems was based on current industrial practice and reports involving the selective extraction of base metals from ore. Sherritt Gordon Mines Ltd. in the 1950’s developed an ammonia (NH3) pressure-leaching system to extract Co and Ni from lateritic ores. Pressure leaching of sulfides has been used to extract Co and Ni as aqueous sulfates. The Bureau of Mines has studied O2-chloride pressure leaching as a means of selectively extracting base metals as aqueous chlorides from complex sulfide ores.
Experiments for all three reagent systems used a 0.5-L Parr shaker-type hydrogenation reactor. The rubber-stopped glass reaction bottle had inlets for a thermocouple well and a glass O2 inlet tube. The bottle was rocked in a 3-in arc at 225 c/min. A heating mantle with a temperature controller was used to maintain constant reaction temperature. Maximum recommended pressure and temperature for this system was 50 psig and 125° C.
Ammonium Salt-Oxygen Leaching
Experiments to investigate O2 pressure leaching with ammonium salt solutions to selectively extract Co and Ni were initially performed on IN-738 scrap. Reaction temperatures ranged from 75° to 125° C and reaction times ranged from 2 to 20 h. Tests were conducted at an O2 pressure of 50 psig and with 12 mol NH3 per mole of scrap, based on the assumption that the scrap was all Ni. Solutions of ammonium chloride (NH4Cl), ammonium carbonate [(NH4)2CO3] ammonium hydroxide (NH4OH), and ammonium sulfate [(NH4)2SO4] were tested. Twenty- four experiments were conducted, using the ammonium salt solutions by themselves or in combinations.
Best results from leaching IN-738 scrap are listed in table 6. Cobalt and nickel were incompletely extracted, and significant amounts of Mo, W, and Zn were also dissolved. Similar results were obtained from leaching B-1900 + Hf scrap. Because of incomplete leaching of Co and Ni over a wide range of experimental conditions and
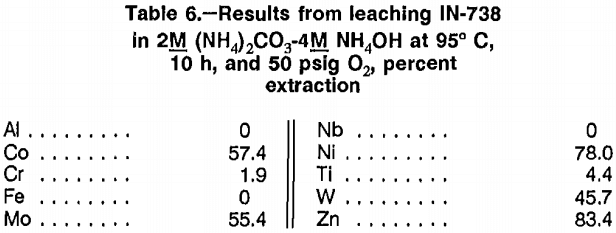
lack of desired selectivity, this method was not promising enough to warrant further investigation.
Acid-Oxygen Leaching
A series of experiments was performed with H2SO4 or HCl as leachants to determine which acid would be more effective in leaching superalloy scrap. Acid-O2 pressure-leaching experiments were conducted at 95°, 105°, and 125° C, with reaction times from 1 to 8 h. In each experiments, 10.0 g of scrap was leached. Acid stoichiometry was calculated assuming Al, Cr, and Fe metals would react to form trivalent aqueous species and Co, Cu, Mn, Ni, and Zn would react to form divalent aqueous species. Calculation of amounts of acid required was based on values shown in tables 1 and 2.
A typical experiment was conducted as follows: Scrap, water, and concentrated acid were charged into the reaction bottle. The system was pressurized with O2 to 50 psig while the solution was heated. Hydrogen gas that formed as a result of the acid-metal reaction was periodically bled off during heating, and the system was refilled with O2 to the desired pressure. During experiments in which the system was not pressurized with O2, H2 was bled off during heatup.
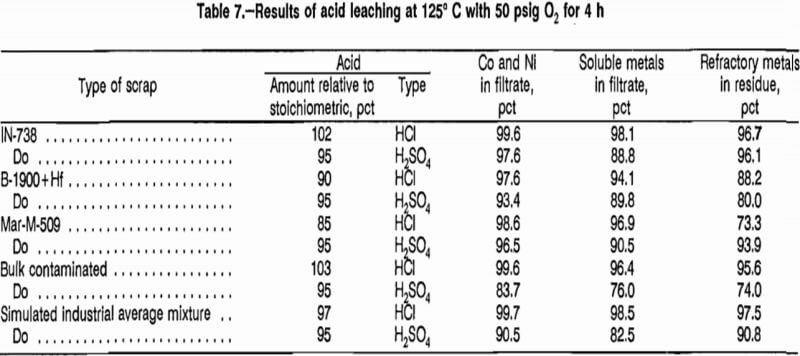
The objective of acid-O2 leaching was to dissolve the maximum amount of Al, Co, Cr, Cu, Fe, Mn, Ni, and Zn from the scrap (this group of metals will be referred to as the soluble metals), while rejecting the maximum amount of Hf, Mo, Nb, Ta, Ti, W, and Zr to the residue (this group of metals will be referred to as the refractory metals). Particular attention was paid to the amount of Co and Ni dissolved. Table 7 lists the best results from these experiments for the five types of scrap leached with HCI or H2SO4 at 125° C and 50 psig O2 for 4 h. Data in table 7 show that HCl was more effective than H2SO4 in leaching Co and Ni from the five types of scrap and gave better separation between the soluble and refractory metals.
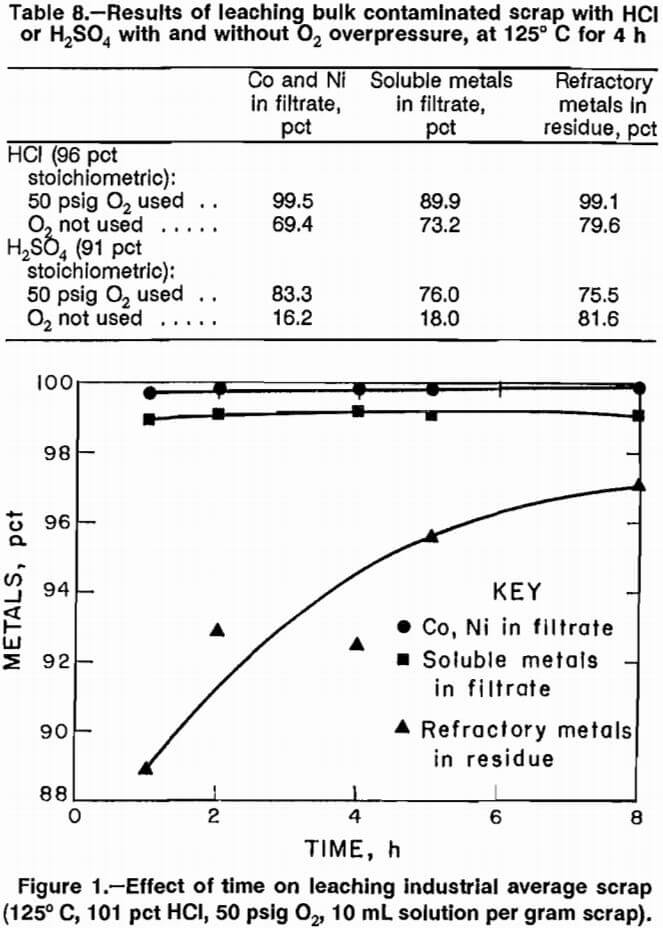
The effect of excluding O2 during scrap leaching was investigated. Tests were performed with HCl and H2SO4 at 125° C, with and without O2 overpressure. Results of experiments using bulk contaminated scrap (table 8) show that the presence of O2 was beneficial. Results for the other four types of scrap followed the same trend, but did not show a dramatic difference between tests performed with and without O2 overpressure.
It was decided to concentrate additional work on scrap leaching in a HCl-O2 system. Simulated industrial average scrap mixture was used to investigate the effects on selectivity exerted by leaching time, solid-liquid ratio, O2 pressure, acid stoichiometry, and temperature.
Leaching time was varied from 1 to 8 h to investigate its effect on selectivity. Experimental conditions were fixed at 125° C, 101 pct stoichiometric HCl, 50 psig O2, and 10 mL of solution per gram of scrap. Data plotted in figure 1 show that 1 h is sufficient to dissolve the Co, Ni, and other soluble metals, but longer leaching times are needed to allow the initially dissolved refractory metals to form solid species and be rejected to the residue. A leaching time of 4 h was selected as the best reaction time based on a compromise to maximize the amount of refractory metals rejected to the residue and to minimize leaching time. Improved reactor design could decrease the time required to leach superalloy scrap. Smyres showed that leaching times for sulfide concentrates decreased by a factor of 6 to 8 when a 50-gal stirred reactor was used rather than a 0.5-L shaker bottle.
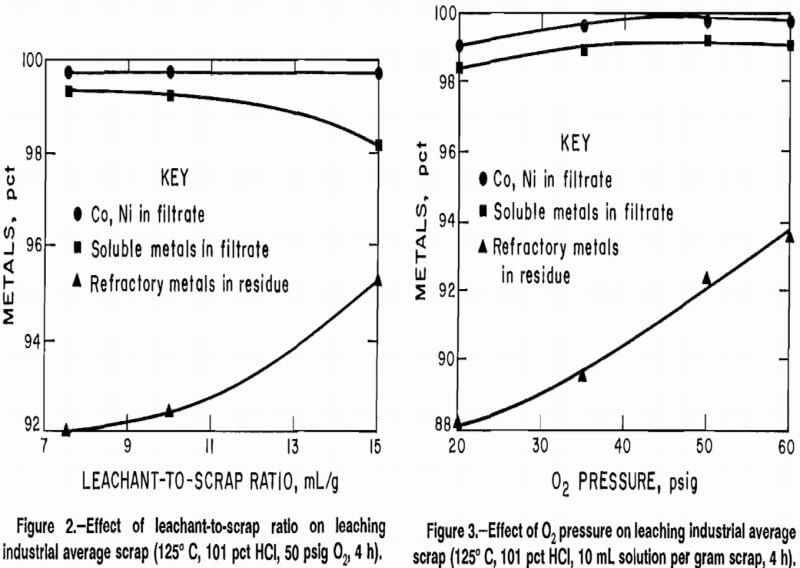
The effect of the ratio of solution volume to scrap weight on separation was determined. Solution volume was assumed equal to the sum of the volume of concentrated HCl and the volume of distilled water. For these experiments the amount of acid remained constant, but the amount of water was varied. Results for tests conducted at 125° C, 101 pct stoichiometric HCl, 50 psig O2, and a leaching time of 4 h are shown in figure 2. The major effects of changing the volume-weight ratio were that an
increase in the ratio resulted in an increase in the amount of refractory metals rejected to the residue and a decrease in the concentration of the metals in the leach filtrate, owing to increased solution volume. A ratio of 10.0 mL of solution to 1.00 g of scrap resulted in good extration of the soluble metals and good rejection of the refractory metals, and produced a solution of sufficiently high concentration for subsequent treatment.
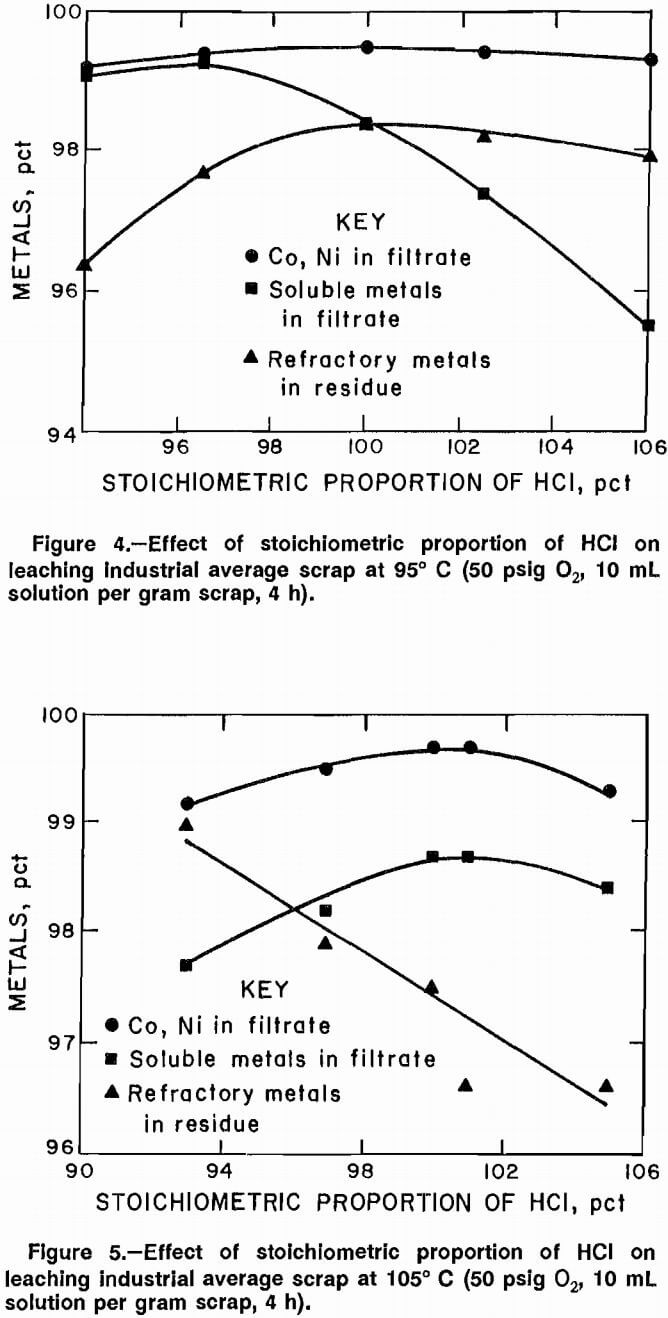
The effect of O2 pressure on scrap leaching was investigated at 125° C, 101 pct stoichiometric HCl, 10 mL of solution to 1 g of scrap, and a leaching time of 4 h. Oxygen pressure was varied from 20 to 60 psig. Data plotted in figure 3 show that increased 02 pressure resulted in a small increase in the amount of Co, Ni, and soluble metals leached. The amount of refractory metals rejected to the residue increased with higher O2 pressure. Owing to limited capability of the experimental equipment, only one test at 60 psig was performed and pressures above 60 psig could not be investigated. The maximum pressure at which a large number of experiments could be safely performed was 50 psig.
The effect of acid strength on selective leaching of the scrap was investigated. Acid strength was measured as percent stoichiometric HCl required and was varied from 94 to 106 pct. Experiments were conducted at 95°, 105°, and 125° C, 50 psig 02, 10 mL of solution to 1 g of scrap, and leaching time of 4 h. The data are plotted in figures 4, 5, and 6. Results do not clearly show a best acid concentration based on maximum soluble metal dissolution and refractory metal rejection. Since there is little variation in the amount of Co and Ni dissolved over the range of acid stoichiometry from 97 to 100 pct, a decision on
what quantity of acid to use would be based on the leaching temperature selected and the relative economic value of the soluble metals dissolved and the refractory metals rejected to the residue.
Data plotted in figures 4, 5, and 6 also show the effect of leaching temperature on selective leaching of scrap. Increasing the leaching temperature from 95° to 125° C slightly increased the amount of soluble metals dissolved.
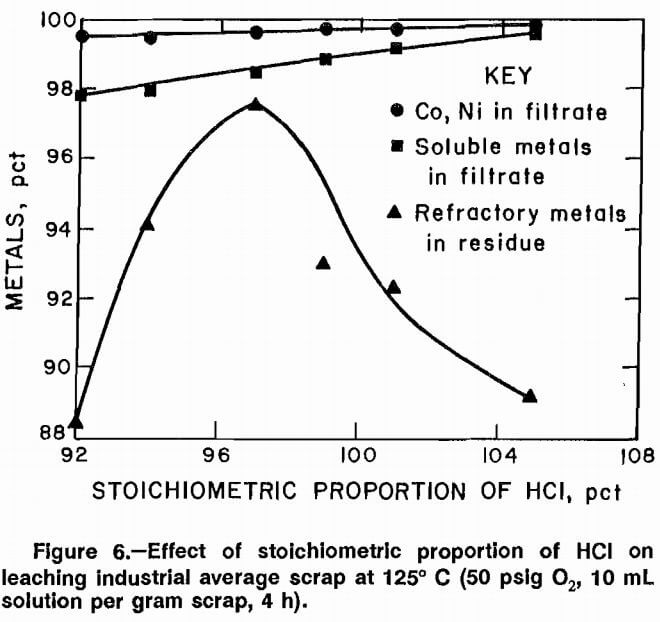
However, the major effect was that smaller amounts of the refractory metals were rejected to the residue at higher temperatures. Conditions of 95° C and 100 pct stoichiometric acid were selected as the best compromise.
After the specific parameters were investigated, a series of 10 experiments was performed using a larger, 2.0-L reaction bottle, with the best conditions determined from previous tests: 95° C, 100 pct stoichiometric HCl, 50 psig
O2 10 mL of solution to 1 g of scrap, and leaching time of 4 h. The same experimental procedure was used as in tests with the 0.5-L reaction bottle, except the charge was increased to 700 mL of solution and 70.0 g of simulated industrial average scrap. This produced a stock solution for use in subsequent solution treatment tests. Average results for the 10 experiments are listed in table 9. There was a ± 1 pct variation in the results for these experiments.

Chlorine-Oxygen Leaching
Using concentrated HCl permitted the accurate addition of cl- to the small amount of leaching solution employed but would create problems in scaleup because of the cost of HCl and the generation of H2. It is proposed that the final recovery of Co and Ni from purified solutions would be accomplished by electrowinning. This method would deposit pure metal on the cathode and generate Cl2 gas at the anode. Chlorine recycling and decreased O2 consumption would be realized if Cl2 gas could be substituted for HCl. Tests were performed to evaluate substitution of Cl2 for HCl using conditions equivalent to those of the HCl tests. Using the 2.0-L reactor allowed the amount of Cl2 introduced to be accurately monitored.
The 70 g of scrap and 700 mL of water were charged into the 2.0-L reactor. The reactor was flushed with O2, Cl2 was turned on, and the temperature was increased. No bleeding of pressure from the reactor was required in these tests because H2 was not generated. Extent of Cl2 addition was determined by monitoring the weight change of the lecture-bottle-size gas cylinder. A 93.8-g weight change indicated 100 pet stoichiometric Cl2 addition. After Cl2 addition, O2 was suppplied to the reactor at 50 psig. Reaction temperature was varied from 95° to 125° C and leaching time from 4 to 24 h after Cl2 addition was complete.
Consumption of Cl2 was self-limiting and temperature dependent. At 95° C, 98.6 pct of stoichiometric Cl2 was the maximum amount consumed. Under these conditions the best results were the dissolution of 95.2 pct Ni and Co and 88.6 pct of the solubles and rejection of 90.8 pct of the refractory metals.
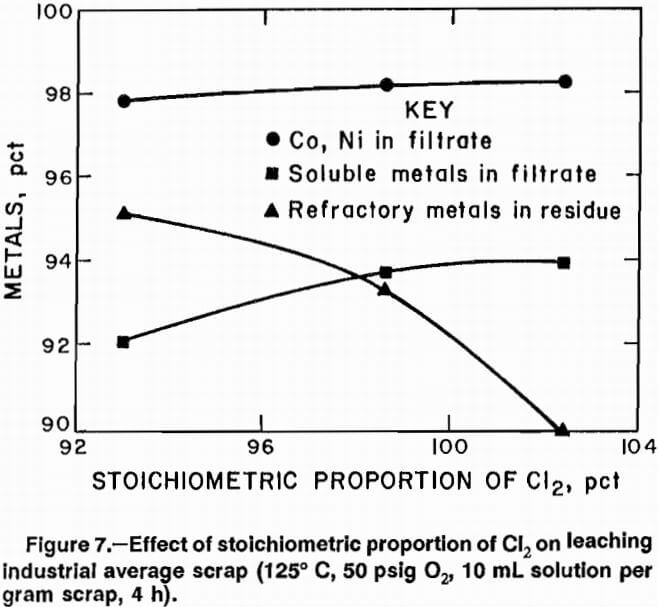
Increasing reaction temperature to 125° C increased the maximum Cl2 consumption to 102.4 pct of stoichiometric. Figure 7 shows results of tests conducted for 4 h at 125° C at different percentages of stoichiometric Cl2 consumption. Best results were obtained at Cl2 consumption of 98 pct.
Chromium dissolution decreased from 97 pct when scrap was leached with HCl at 125° C to 77 pct when it was leached with Cl2 at 125° C; this accounts for the decreased amount of solubles dissolved. Refractory metals rejection was lower with Cl2 than with HCl, but the refractory metals remaining in solution would be removed during second-stage treatment of the filtrate. Advantages of Cl2 over HCl are that it can be recycled from electrowinning to the first-stage leaching step and it cannot form an explosive mixture in the reactor as the H2 from HCl-metal reaction could. A disadvantage is that Cl2 leaching requires a longer time. HCl tests required a total of 4 h, while Cl2 tests required 4 h to introduce Cl2 into the system and additional time to complete the reaction.
Flowmeter-Gas Chromatography
Oxygen consumption during HCl and Cl2 leaching experiments was determined. A recording mass flowmeter, calibrated for O2, was installed in the inlet side of the reactor’s flow system. A check valve protected the O2
flowmeter’s transducer from corrosion by Cl2 during intervals when Cl2 was added to the reactor. A sample of the reactor atmosphere was taken each time gas was released from the system. These samples were analyzed with a gas chromatograph to determine O2 concentrations. Hydrogen concentrations were determined by difference.
Refilling the reactor after bleeding H2 required a flow rate beyond the mass flowmeter’s capability. Ideal gas calculations and gas chromatograph analyses of the offgas were used to determine 02 and H2 balances.
Calculations showed that, for treating 1 g of simulated industrial average scrap, a total of 0.17 L O2 was consumed during HCl leaching compared with 0.004 L O2 during Cl2 leaching. A mass balance on the HCl system was performed for H2 and O2. Calculations based on treatment of 1 g of simulated industrial average scrap showed that 0.40 L H2 was generated from metal-acid reactions, 0.24 L O2 was charged to the system, and the total bleed gas contained 0.11 L H2 and 0.07 L O2. Apparently, part of the 0.17 L O2 that was consumed per gram of scrap oxidized the refractory metals, and the balance reacted with the 0.29 L H2 not bled to form H2O. It was not possible to measure changes in water volume to confirm that the assumed reaction of H2 and O2 to form water had occurred.
Observation of the mass flowmeter record during HCl leaching indicated that the leaching reaction was impeded when the O2-H2 ratio was small. After the reactor reached the desired temperature and pressure, another bleed of H2 overpressure and backfill with O2 were needed to initiate a renewed demand for O2. Gas chromatograph analysis of the offgas showed that an O2-H2 ratio greater than 1:2 was necessary to make the reaction proceed rapidly.
Atomized Scrap Leaching
Atomized scrap was leached with HCl-O2 in the 0.5-L bottle reactor for comparison with Zn-treated scrap leached under similar conditions. Experimental procedure and acid stoichiometry calculations for tests using atomized scrap were identical to those for Zn-treated scrap leaching experiments. Acid stoichiometry calculations were based on values in table 3. Temperatures of 95°, 105°, and 125° C, leaching times of 1, 2, and 4 h, and acid strengths of 97, 100, and 103 pct of stoichiometric HCl were investigated.
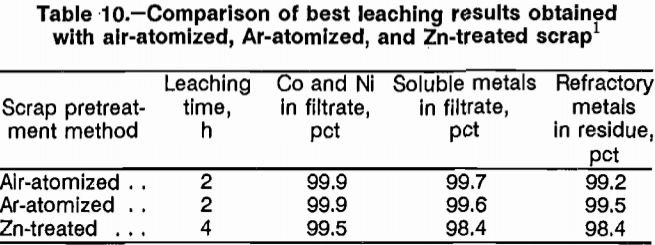
Results showed no discernible difference in leachability between air-atomized and Ar-atomized scrap; identical results were obtained when atomized scrap was leached at 95° and 105° C. Results obtained by leaching atomized scrap at 95° or 105° C were slightly better than results obtained by leaching Zn-treated scrap. Leaching atomized scrap at 125° C was not as effective in dissolving soluble metals and rejecting refractory metals to the residue as was leaching at lower temperatures. Best results for leaching air- and Ar-atomized scrap were obtained by leaching with 100-pct stoichiometric HCl at 95° C for 2 h using 50 psig O2. Results at these conditions are listed in table 10. Best results for Zn-treated scrap are also listed in table 10 for comparison.
Tests were performed to determine whether Cl2 could be substituted for HCl to leach atomized scrap. Experimental procedure for these experiments was identical to that used for Cl2-O2 leaching of Zn-treated scrap. Results from these experiments showed that Cl2-O2 did not leach either air-atomized or Ar-atomized scrap at 95° or 125° C.
Aluminum-Chromium-Iron Precipitation From HCl-O2 Pregnant Solution
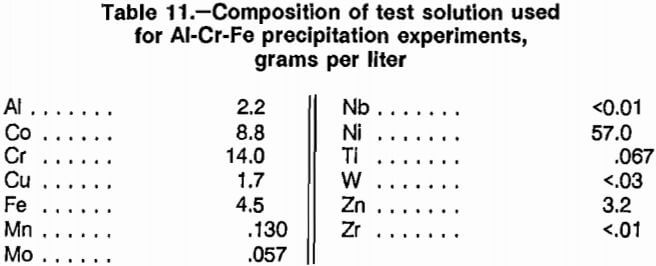
Leaching of superalloy scrap with HCl-O2 followed by filtration resulted in a residue containing most of the refractory metals concentrated by a factor of -6 and a solution containing 99.2 pct of the Co and Ni and other soluble elements. The solution used for the experiments was from the series of 10 HCl-O2 scrap leaching experiments conducted with the 2.0-L reaction bottle (table 9). The composition of the test solution is given in table 11.
Three techniques were investigated to selectively precipitate Al, Cr, Fe, and other impurities from first-stage pregnant solutions while minimizing Co and Ni loss. The techniques were (1) precipitation of hydroxides at room temperature, (2) scavenger metal-O2 treatment, and (3) hydrothermal precipitation.
Room-Temperature Hydroxide Precipitation
Precipitation of Al, Cr, and Fe as hydroxides by pH adjustment with the addition of NaOH solution was tested at ambient conditions. While 75 mL of test solution was stirred, 5.2M NaOH was added until the pH reached a predetermined value. After the precipitate had settled, the solution was filtered. Filtration of the solution was difficult because of the gelatinous nature of the precipitate. Adjustment of the solution pH to 3.7 resulted in precipitation of 96 pct of the Al, Cr, and Fe, and 24 pct of the Co and Ni. Vacuum filtration of the 75 mL of solution took approximately 16 h using a 9.0-cm Buchner funnel. Experiments using this technique to remove Al, Cr, and Fe from solution were discontinued because of excessive Co and Ni losses and the long filtration time.
Scavenger Metal-Oxygen Treatment
Experiments were conducted in which pure Cu, Ni, or Zn metal powder was added as a scavenger metal to precipitate Al, Cr, and Fe from solution. Copper, nickel, and zinc were chosen for investigation as scavenger metals because they would be present in solution after Al, Cr, and Fe are removed. Scavenger metal additions were based on equation 1, where M represents Al³+, Cr³+, or Fe³+. Stoichiometric coefficients remain the same if Cu or Zn is substituted for Ni as the scavenger metal.
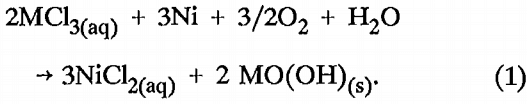
Experiments were performed in the 0.5-L shaker-bottle apparatus used for the O2 pressure-leaching experiments and with 75 mL of test solution. An experiment performed at 93 pct of stoichiometric Ni, 50 psig O2, and 125° C for 4 h resulted in 96.6 pct of the Co and Ni remaining in solution and 93.8 pct of the Al, Cr, and Fe precipitating. Vacuum filtration of 85 mL of solution took approximately 18 h using a 9.0-cm Buchner funnel.
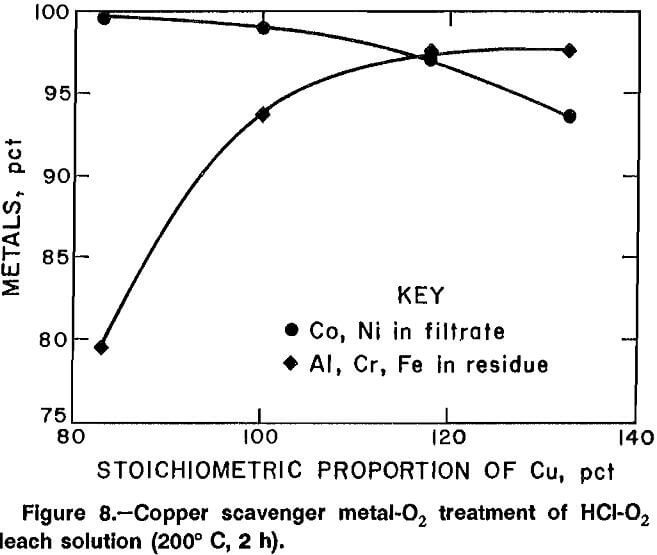
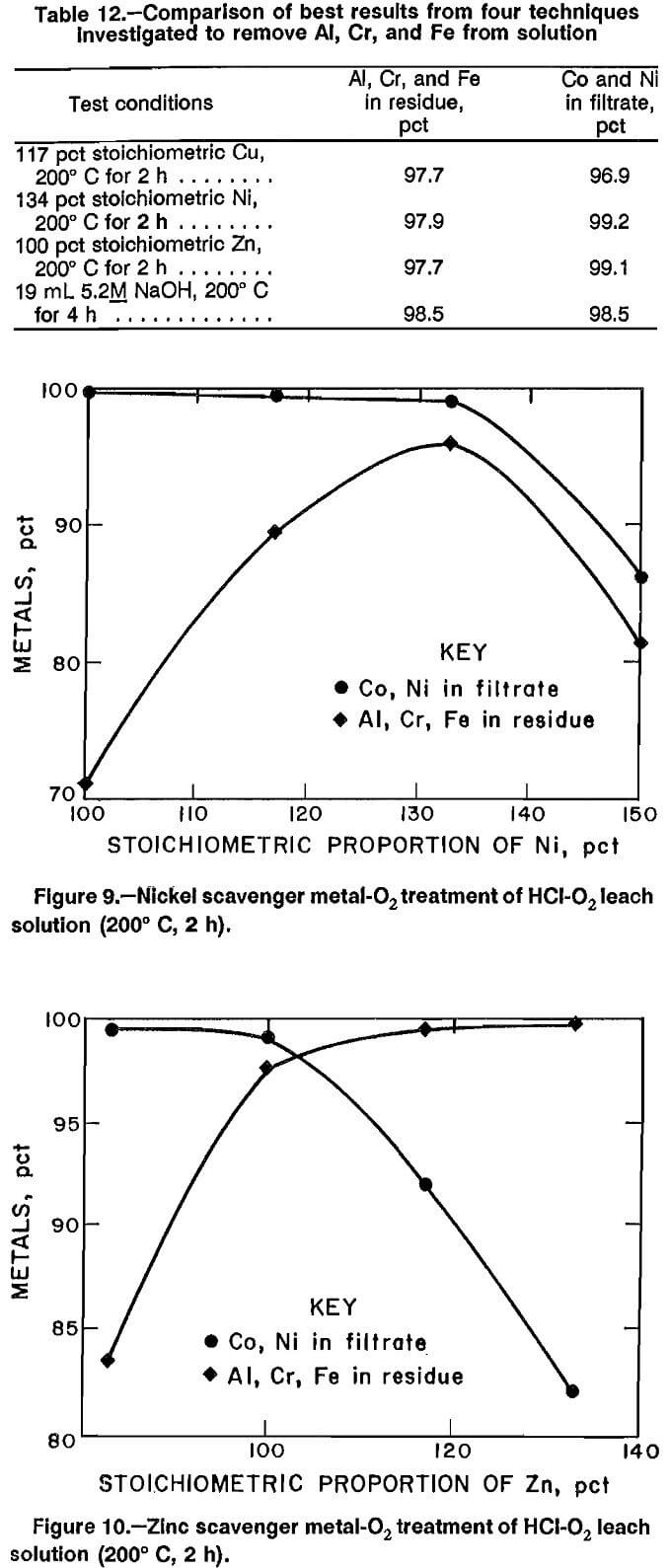
In an attempt to increase the filtration rate, experiments were conducted in a high-temperature autoclave lined with polytetrafluoroethylene (PTFE). This permitted investigation of temperatures up to 215° C. When the reaction temperature was increased above 125° C, the Al, Cr, and Fe formed a readily filterable precipitate. Stoichiometry for the scavenger metal addition was based on equation 1. Oxygen pressure was set at 725 psig, and the solution was heated to 200° C and held at temperature for 2 h. Results for Cu, Ni, and Zn scavenger metal experiments are plotted in figures 8, 9, and 10, and the best results for each scavenger metal are listed in table 12, along with results of a hydrothermal precipitation method. The results show a tradeoff between the amount of Al, Cr, and Fe precipitated and the amount of Co and Ni remaining in solution. Vacuum filtration of 85 mL of solution took less than 1 h using a 9.0-cm Buchner funnel.
Hydrothermal Precipitation
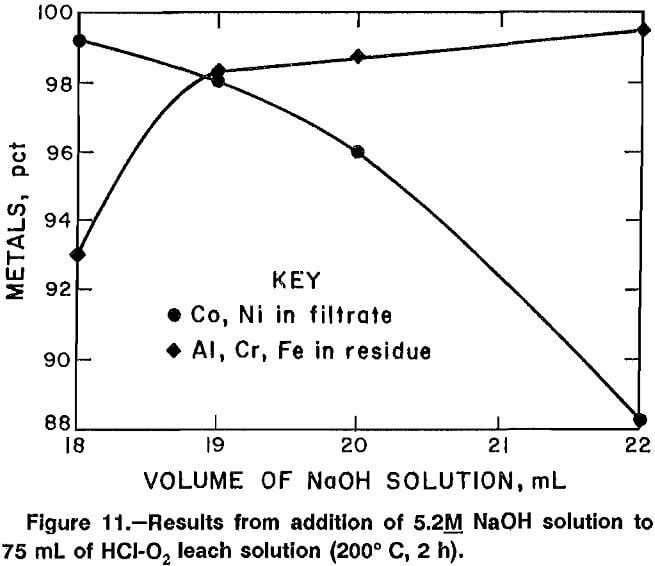
Experiments were conducted to investigate the effects of adjusting the pH of the test solution with a base and heating the solution in the PTFE-lined autoclave. Temperatures were varied from 160° to 215° C, and reaction times of up to 4 h were used. “Reaction time” refers to the length of time the solution was held at elevated temperature. Either a specific volume of 5.2M NaOH was added to the test solution or the pH was adjusted to a predetermined value. In either case, the solution was continuously stirred while base was added. Each experiment used 75.0 mL of test solution.
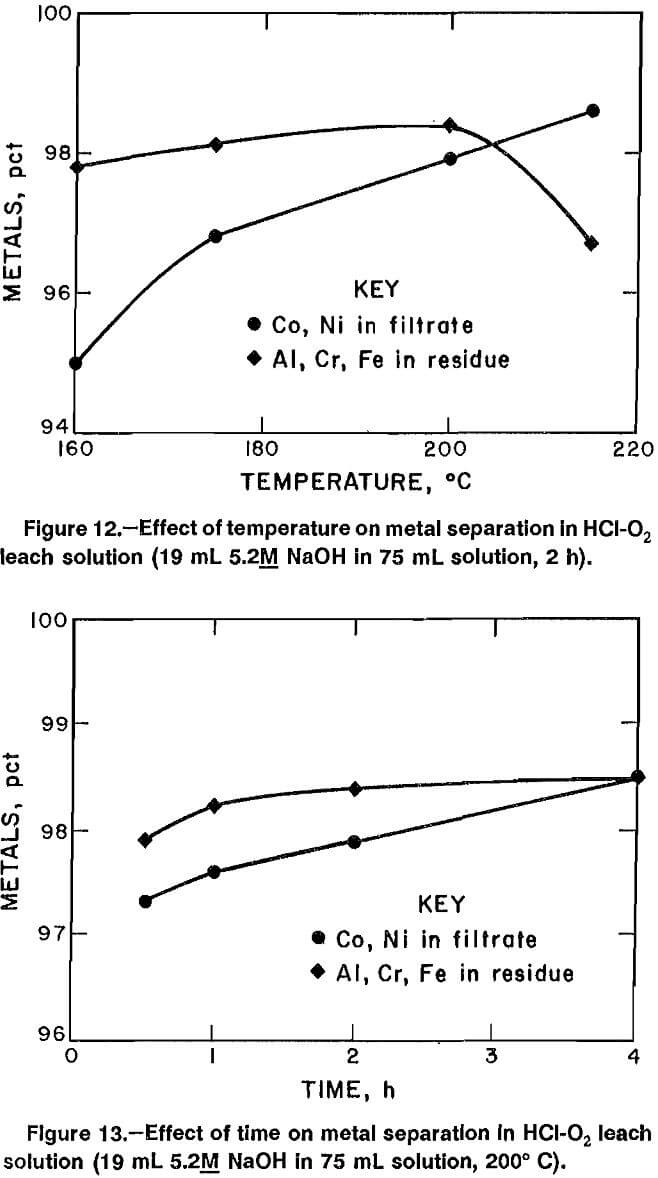
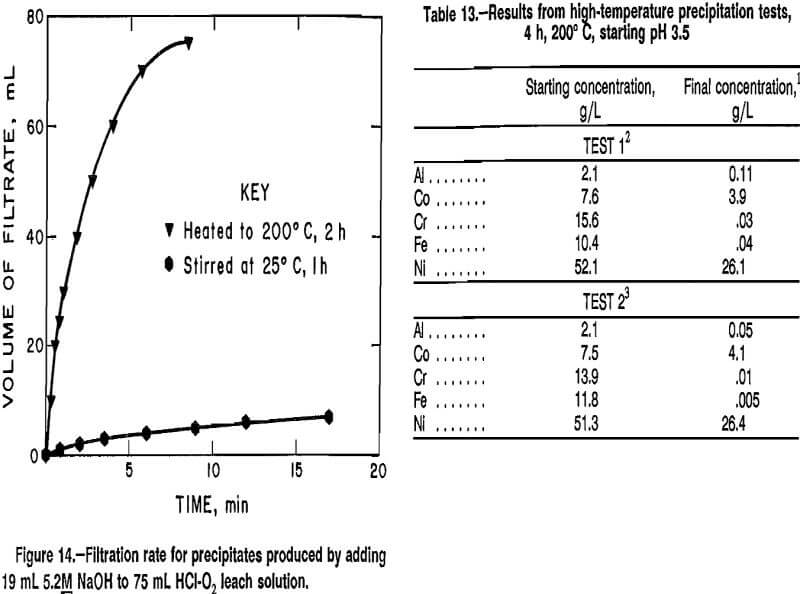
Data from experiments in which the amount of 5.2M NaOH was varied are plotted in figure 11. Addition of 19.0 mL of solution produced the best combination of precipitating Al, Cr, and Fe and maintaining Co and Ni in solution. Adding 19.0 mL of 5.2M NaOH increased the pH to 3.45, and heating the solution to 200° C for 2 h resulted in precipitation of 98.4 pct of the Al, Cr, and Fe, with 97.9 pct of the Co and Ni remaining in the filtrate. The same volume of hydroxide solution was added in other experiments designed to determine the best reaction temperature and time. Data from experiments of 2-h duration in which the reaction temperature was varied between 160° and 215° C are plotted in figure 12. Data from experiments performed to determine the best reaction time are plotted in figure 13. The best reaction time was determined to be 4 h.
Filtration rate data for precipitates produced at 200° C and at room temperature were obtained. Experiments consisted of adding 19.0 mL of 5.2M NaOH to 75.0 mL of test solution. One sample was heated to 200° C for 2 h in the PTFE-lined autoclave, whereas another sample was stirred in an open beaker at room temperature for 1 h. The solutions were filtered with a 9-cm Buchner vacuum filtration apparatus. A graduated cylinder was placed in the filter flask to measure filtrate volume as a function of elapsed time. Data are presented in figure 14. Compared with the precipitate produced at room temperature, the precipitate produced at 200° C filtered about 50 times faster.
Comparison of Scavenger Metal-Oxygen Treatment and Hydrothermal Precipitation
The best results from the use of three different scavenger metals and the hydrothermal precipitation method are shown in table 12. The four techniques are effective in precipitating Al, Cr, and Fe, while minimizing Co and Ni losses, but the use of scavenger metals has serious drawbacks. The main problem is that Cu and Zn scavenger metals dissolve during the process and must be removed during a later step. Results from experiments later performed to remove Zn from solution showed that the methods of removal investigated were ineffective, and therefore, Zn contamination is unacceptable. Removal of the Cu by sulfide precipitation would also remove large amounts of Co and Ni and result in loss of those elements. The problem with using Ni as the scavenger metal is that Ni is the most expensive metal of the three tested and must be used in the greatest stoichiometric amount.
The hydrothermal precipitation technique does not require an analysis of the solution. If the pH of the solution is monitored while base is being added, a solution can be produced with low levels of Al, Cr, and Fe. The test solution used for all previous tests was altered for two experiments by adding chromic or ferric chloride (CrCl3 or FeCl3). Without an analysis of the solution, base was added to raise the pH to 3.5, and the solution was heated to 200° C for 4 h. Results from these two tests are listed in table 13. Minor amounts of refractory metals that were in solution after the first-stage leach also reported to the residue, and the concentrations were less than 0.03 g/L for W and less than 0.01 g/L for the other refractory metals in the solution.
Solution Purification
The filtrate from the hydrothermal hydroxide precipitation step had a pH of about 1.6 and contained minor amounts of Al, Cr, Fe, Cu, and Zn at levels unacceptable for a process of Co-Ni solvent extratction and electrowinning. Increasing the pH and adding sulfide ion were tested as methods to remove all remaining impurities. Since the amount of filtrate available was small, a synthetic solution containing impurities in known amounts was prepared for testing and the results were confirmed on a genuine solution. Experiments were conducted at room temperature on 100-mL samples in a stirred beaker. Sulfide additions were made with 1.3M Na2S (sodium sulfide), and pH was adjusted with 5M NaOH.
Increasing the pH to 4.5 removed the remaining Al, Cr, and Fe to less than 0.03 g/L in solution. The small amount of precipitate could be recycled to the leaching step to recover coprecipitated Co and Ni. Copper concentration was decreased to 0.027 g/L by addition of 50 pct excess sulfide ion as Na2S. As much as 3 pct of the Co and Ni in solution was coprecipitated with the copper sulfide (CuS). Copper would be in the solution only if it were accidentally present in a batch of superalloy scrap, e.g., a piece of Monel or other such alloy. Copper removal by sulfide precipitation and the loss of Co and Ni would be required only if Cu were detected in the solution at this stage.
Less than 10 pct of Zn was removed from solution by pH adjustment or sulfide precipitation. The best method for controlling Zn in solution is to remove all Zn from the scrap before first-stage leaching. Use of higher temperatures for vacuum distillation of Zn decreases the Zn content of the scrap. In the work cited, samples of simulated industrial average scrap were redistilled under 30 µm vacuum for 4 h at 950° and 1,000° C. Zinc content was lowered from 3.1 pct to 0.033 pct and 0.011 pct, respectively. Leaching either residue with 100 pct stoichiometric HCl at 95° C for 4 h with 50 psig O2 dissolved 99.6 pct of the Co and Ni and 98.2 pct of the soluble metals, and rejected 96.8 pct of the refractory metals to the residue. Zinc levels for these two solutions were below the analytical detection limit of 0.003 g/L.
The purified Co-Ni solution is suitable feed for solvent extraction separation of Co and Ni prior to electrowinning to produce pure metal.
It was not possible to perform solvent extraction and electrolysis experiments to demonstrate this because of budget and time constraints. A number of publications report results on separation and electrolysis of Co and Ni from chloride solutions.
Caustic Fusion of Oxygen Pressure-Leached Residue
The refractory metals (Hf, Mo, Nb, Ta, Ti, W, and Zr) initially in the scrap were concentrated by a factor of 6 in the residue during the HCl-O2 leaching process. Results already listed (table 9) show that an average of 98.2 pct of the refractory metals reported to the HCl-O2 leached residue for 10 experiments performed in the 2.0-L reactor. The values in table 2 for the calculated composition of simulated industrial average superalloy scrap show that the refractory metals make up 6.4 pct of the total scrap. Data in tables 2 and 14 show that these metals represent 22.9 pct of the total dollar value of the scrap in terms of the pure constituent metals.
Exploratory experiments were conducted to investigate the extraction of Mo and W from O2 pressure-leached residue. Selection of methods to separate the metals for marketing would depend on the amount of each metal in the actual residue and its value and market demand at the time of treatment. Material used for these experiments was produced during the 10 HCl-O2 experiments conducted in the 2.0-L reactor. Composition of this material is given in table 15. Experiments consisted of placing approximately 1.3 g of the residue and a specific amount of NaOH in an iron crucible and heating to above the melting point of solid NaOH for a predetermined length of time. After the crucible was removed from the furnace and allowed to cool, the fused material was leached with hot water (90° C) and water at room temperature. Reaction temperatures investigated ranged from 400° to 700° C, and time at temperature ranged from ½ to 4 h. Duration of leaching with water was also varied.
Experiments were initially performed at a fusion temperature of 400° C to selectively extract Mo and W from the residue. A fusion time of 4 h and a NaOH-to-residue weight ratio of 3.5 resulted in extraction of 88 pct of the Mo and W and rejection of 97 pct of the other metals to the residue. Increasing the fusion temperature to 700° C gave increased Mo and W extraction. The increase in Mo and W extraction came at the expense of increased Cr extraction. An experiment performed at 700° C for 4 h and at a NaOH-to-residue ratio of 7.0 extracted greater than 99 pct of the Mo and W and 97 pct of the Cr, and rejected 97 pct of the other metals to the residue. Leaching at 90° C did not increase Mo, W, or Cr extraction and did not increase rejection of the other metals to the residue relative to leaching at room temperature.
Chromium-Iron Reduction
The precipitate from hydrothermal precipitation tests was treated to investigate recovery of Cr and Fe as a ferrochromium alloy. Carbon and silicon were investigated as reductants. The experimental procedure involved mixing the precipitate with CaO (lime), CaF2 (calcium fluoride), and C or Si, placing the mixture into graphite or alumina crucibles, and heating to 1,620° C.
Carbon reductant in graphite crucibles gave poor results. CO produced by reaction of C with metal oxides caused much of the material in the crucible to splash up onto the crucible walls. Even though metal and slag phases formed, accurate weights of either phase could not be obtained. The molten metal also had a tendency to wet the walls of graphite crucibles, which further distorted the results. Since the purpose of this portion of the project was to show the feasibility of treating this material and not to determine best conditions, Si was used as the reductant rather than C even though it may be uneconomical for use on a large scale.
Experiments were performed with Si as the reductant, in alumina crucibles, and with CaO and CaF2 as slagging reagents. A mixture of 57.3 pct precipitate, 19.0 pct Si, 17.0 pct CaO, and 6.7 pct CaF2 was heated to 1,620° C for 15 min. A head analysis of the precipitate is listed in table 16. The resulting metal phase formed a buttom that contained 98.6 pct of the Cr, 98.5 pct of the Fe, and 99.7 pct of the Ni. The slag contained 99.2 pct of the Al.
Proposed Leaching-Purification-Electrowinning Procedure
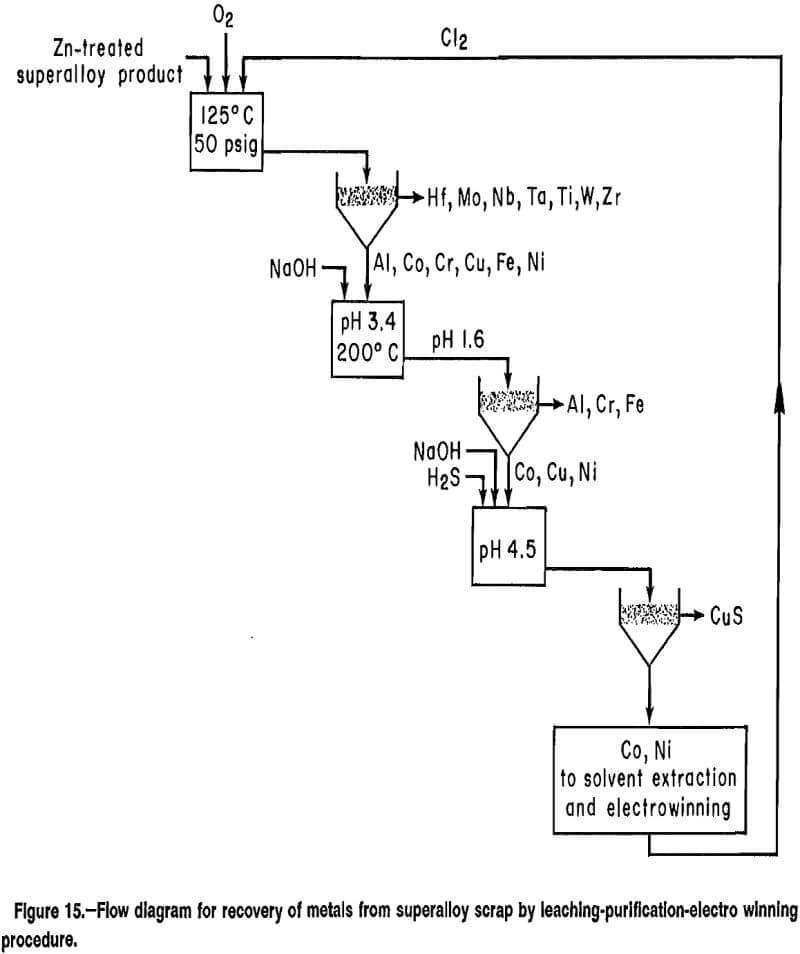
A flow diagram for a proposed leaching-purification-electrowinning process based on the experimental results is presented in figure 15. Zinc-treated superalloy scrap is leached with 02 and recycled Cl2 at 125° C to selectively dissolve Al, Co, Cr, Cu, Fe, and Ni. Aluminum, chromium, and iron are precipitated from solution by adjusting the pH with a hydroxide solution and heating to 200° C. Minor amounts of Al, Cr, Cu, and Fe are removed at ambient conditions with hydroxide and sulfide addition. Cobalt and nickel are separated by solvent extraction, and electrowinning produces pure Co and Ni metal and Cl2 for recycle.
Summary
Methods were investigated to recover Co and Ni from mixed contaminated superalloy scrap. Leaching Zn-treated scrap with HCl-O2 at 95° C dissolved over 99 pct of the Co and Ni and greater than 97 pct of the Al, Co, Cr, Cu, Fe, and Ni, and rejected greater than 98 pct of the Mo, Nb, Ta, Ti, W, and Zr to the residue. Chlorine gas can be substituted for HCl with only a slight decrease in effectiveness, but leaching temperature must be increased to 125° C. Advantages of using Cl2 gas instead of HCl are that Cl2 can be recycled from the electrolysis cells, H2 gas production during pressure leaching is eliminated, the amount of Cl2 used is regulated by the leaching reactions (no analysis is required to calculated stoichiometric requirements), and 02 consumption is decreased from 0.17 L to 0.004 L per gram of scrap treated.
Leaching air- or Ar-atomized scrap with HCl-O2 at 95° C dissolved 99.9 pct of the Co and Ni and over 99 pct of the Al, Cr, Cu, and Fe, and rejected over 99 pct of the Mo, Nb, Ta, Ti, W, and Zr to the residue. Chlorine gas cannot be substituted for HCl because it is ineffective in leaching atomized scrap.
The removal of Al, Cr, and Fe was achieved by adjusting the pH of the HCl-O2 leach filtrate with hydroxide solution and heating to 200° C.
This technique precipitated 98.5 pct of the Al, Cr, and Fe while coprecipitating only 1.5 pct of the Co and Ni. Hydrothermal precipitation at 200° C produced a readily filterable precipitate.
Filtrate from the Al-Cr-Fe hydrothermal precipitation step was treated with hydroxide and sulfide solutions at ambient conditions to decrease the amounts of Al, Cr, Cu, and Fe to levels low enough to make the solution suitable as feed for Co-Ni separation by solvent extraction and subsequent recovery by electrowinning.
The Al-Cr-Fe precipitate was mixed with flux and Si reductant and heated to 1,620° C. The metal that formed contained 98 pct of the Cr, 98 pct of the Fe, and 99 pct of the Ni initially present. The slag contained 99 pct of the Al initially present.
