Table of Contents
The discovery of rhenium on the ion exchange resin used at the uranium in-situ leach operations at Palangana (Texas) led to a laboratory study on the possible methods of recovery. Rhenium, present in small amounts in the ammonium carbonate leach liquors as the perrhenate anion, ReO4-, concentrated on the anion exchange resin, which was a factor of ten more selective for rhenium than for uranium. Various elution systems were investigated for stripping the rhenium from the columns after the uranium had been eluted with chloride or nitrate solutions.
Occurrence
Rhenium in nature is almost always associated with molybdenite. On a 100% M0S2 basis, molybdenite concentrates from copper deposits in the western United States contain typically 0.02-0.2% Re. Molybdenite itself has never been identified in the ore at Palangana, but previous studies do indicate a highly reactive M0S2 compound such as the amorphous mineral jordisite. The rhenium is probably associated with this phase. The M0S2 is leached very rapidly when in contact with oxidant-bearing ammonium carbonate solution, and the associated rhenium can dissolve at the same time. Thus, it is felt that the Re/Mo ratio in solution is the same as it was in the ore. The same can be said for the Re/U3O8 ratio, since the uranium also is rapidly leached in contact with the oxidizing carbonate system, and U3O8 and Mo leach recoveries are generally >90% from an area which has seen enough oxidant to oxidize UO2 and MoS2.
Rhenium Loading
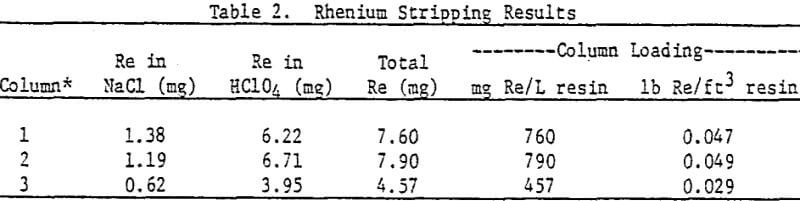
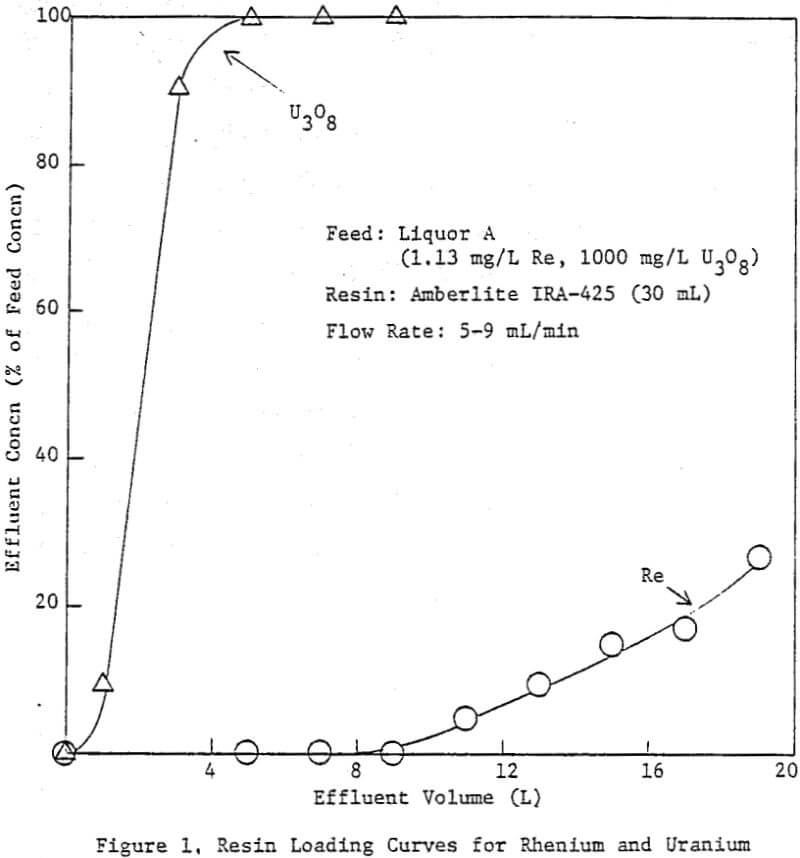
To determine the maximum rhenium loading anticipated from an actual ammonium carbonate leach liquor, three 1.2-cm diameter glass columns were filled with 10 mL each of fresh Amberlite IRA-425 resin (chloride form) and stacked in series. Twenty liters of liquor A was then passed through the columns at 5-9 mL/ min. Effluent was collected in 2-L intervals, and analyzed for uranium and rhenium.
The first two columns appear to have reached an equilibrium loading of rhenium, since they both contained essentially the same quantity (760-790 mg Re/L resin). The third column was loaded to about 60% of the equilibrium capacity. The average uranium loading was 66.7 g U3O8/L resin (4.16 lb U3O8/ft³ resin), typical for fresh Amberlite IRA-425. Thus, the average Re/U3O8 ratio on the resin, fully loaded in both rhenium and uranium, was 0.0116.
A sample of Amberlite IRA-425 resin from the Palangana pilot plant was used to evaluate the effectiveness of various rhenium eluting agents. The resin sample as received had a rhenium loading of 320 mg/L (0.020 lb/ft³). This was about 40% of the equilibrium value determined above. The lower loading here was caused primarily by lower grade feed solutions. The normal uranium elution schedule also prevented the full equilibrium rhenium loading from being attained.
Elution of rhenium from the Palangana resin with 7.5N HCl was incomplete because of rhenium sulfide (Re2S7) precipitation. The sulfide necessary for this precipitation apparently originated from reduced sulfur species such as polythionates which are known to be present on the resin, and which are only partially eluted with brine.
Rhenium Sulfide Precipitation
Rhenium was precipitated from the ion exchange effluents as the sulfide (Re2S7). No sulfide precipitates from neutral solution, and the precipitate tends to remain in a colloidal state in 9N HCl. Rhenium sulfide also may be precipitated from HClO4 solution, although it precipitates more effectively if HCl is added.
Preliminary screening tests confirm that Re2S7 could be precipitated from 2N HClO4, although the precipitate was much slower to form than from HCl. Precipitation occurred rapidly from 2N HClO4-4N HCl solutions, however. Perchloric acid appeared to have little effect on the precipitation. In spite of its strong oxidizing properties, it is chemically inert in these systems.
If most of the nitric acid is substituted by ammonium nitrate (tests 10-12), rhenium sulfide still could be precipitated, although at 0.1N H+, the yield was less. No Re2S7 was precipitated from 3N NH4NO3, even after autoclaving 4 hr at 100°C (tests 13 and 14).
The recoveries from solution were 98 and 96%. The concentrate grades were 7.2-7.6% Re, low principally because of the high elemental sulfur content. The sulfur can form by reaction of some of the H2S with reduced sulfur species eluted from the resin. Also, nitric acid at this low concentration (0.5N H+) may still play a role in H2S oxidation, as well as trace oxidants such as Fe³+.