Table of Contents
The waste-plus-waste treatment method can be successfully used on a variety of acid and alkaline electroplating wastes. The method is economically attractive because of the low cost of the waste-plus-waste addition step: No additional reagents are necessary, and the metal values are concentrated. In the five waste-plus-waste combinations investigated, combination of the two wastes caused almost quantitative precipitation of metals and cyanides. The metal cyanide precipitate contained substantial amounts of valuable metals that were recovered for recycling by a few additional processing steps. The filtrate was relatively harmless compared with the original wastes and meets most water quality standards for cyanide content.
Although the evolution of highly poisonous hydrogen cyanide (HCN) gas was not detected in these tests, equipment for collecting and destroying HCN would be necessary in industrial applications. A ventilated mixing tank with devices for monitoring the cyanide concentration and the pH value of the solution would be required for the waste-plus-waste step.
As many electroplating plants use racks to support small objects and use both nitric acid and alkaline cyanide solutions to strip the racks, the described process could be applied at the source of the two wastes.
As part of its solid waste research program the Bureau of Mines is engaged in studies to develop technically feasible, low-cost processes for recovering valuable materials from a wide variety of industrial plating wastes. Significant tonnages of valuable metals and chemicals can be recovered from these wastes, which are richer in metal content than many ores presently being processed. An additional benefit is the alleviation of the stream and ground water pollution to which these wastes contribute. Many of the waste treatment processes developed in the past have one or more of the following faults; (1) The treatment produces a new waste that also constitutes a disposal problem, (2) the treatment process does not include the recovery and reuse of the metal and other values, or (3) the treatment process is so costly that voluntary adoption by industry is unlikely. The research described herein describes an innovative treatment process for plating wastes that does not have these shortcomings.
The treatment of cyanide wastes to render them innocuous has received extensive coverage in recent years, and those methods have been adequately summarized in three articles. Unfortunately, little that is new has been developed nor have many processes been developed to recover the metal values from these wastes. Conventional methods of destroying cyanides often require equipment which is too costly for small plating shops, and sometimes offer little economic incentive even to large-volume platers. In the treatment of acid wastes, particularly waste pickle liquors, new approaches have been successfully developed to neutralize the sulfuric acid and recover the iron, and to recover iron and hydrochloric acid. There is at least one patent on the recovery of copper from cyanide waste as cuprous cyanide that is somewhat similar to the process described in this report.
Composition of Wastes
Tables 1 and 2 give some examples of current metal losses in electroplating industries; these losses also represent potential profits for future recovery processes. When small articles are electroplated they are supported on racks, and during the plating operations metal is also deposited on the racks. Built-up metal must be removed periodically from the racks by various solutions, which are known as rack-stripping solutions. These solutions are often high in metal values. The annual metal losses in rack-stripping solutions and chromic acid etching solutions from two electroplating companies are given in table 1. The HNO3 waste is hot, concentrated nitric acid, which is used until it will not dissolve any more metal from the racks. Upon cooling, a large amount of precipitate (largely nickel nitrate plus some iron and copper nitrates) appears.
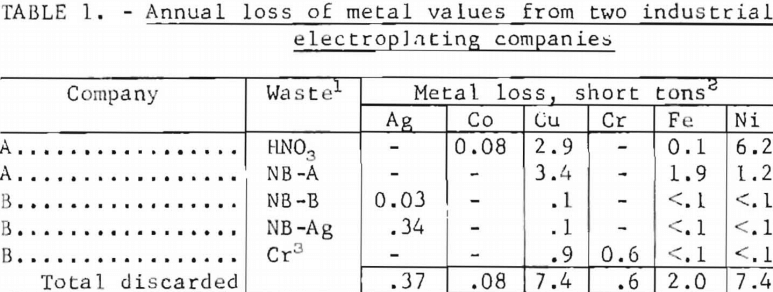
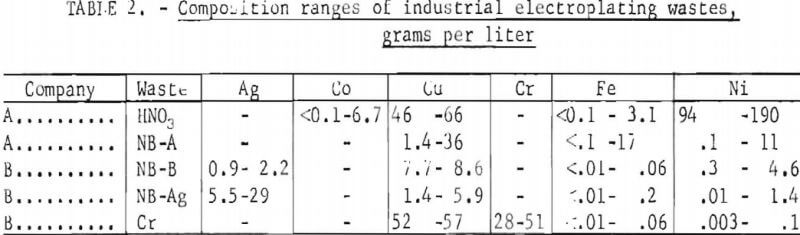
These five wastes from only two companies represent annual losses of approximately 7 tons of nickel, 7 tons of copper, 0.4 ton of silver, and 0.6 ton of chromium. As there are approximately 20,000 electroplating plants in the United States, it is evident that significant amounts of valuable metals are being wasted in these operations. The concentrations of several metals in the same five wastes are given in table 2; the high concentrations of silver, copper, chromium, and nickel make these wastes interesting from an economic viewpoint.
Experimental Procedures and Results
Preliminary test work was carried out on HNO3, rack-stripping solutions. Lime was added to adjust the pH of the solution to 2.3 to 2.7, which caused the precipitation of ferric hydroxide. The precipitate, after filtering and drying at 100° C, contained 36.8 percent iron, 2.4 percent copper, and 0.15 percent nickel. In some tests the copper and nickel in the iron precipitate were recovered by re-solution and re-precipitation. After precipitating iron, the copper was recovered almost quantitatively by controlled potential electrolysis at 0.1 to 0.3 volt. The recovered copper was highly pure; spectrograph analysis revealed only a trace amount of silicon. In other tests, copper was recovered by selective precipitation; additional lime was used to adjust the pH of the solution to 5.5. This precipitate, after being converted to copper sulfate, analyzed 26.6 percent copper and 0.01 percent nickel; no iron was detected.
The remaining solution of nickel nitrate was treated by either of two different methods:
- Additional lime was added to adjust the pH of the solution to 9.5, which caused nickel hydroxide to precipitate. The filtrate from this precipitation was an approximately 1.5 molar solution of calcium nitrate, which has some value as plant food.
- The solution was treated with concentrated sulfuric acid and heated to 100° C to evolve nitric acid and precipitate calcium sulfate, leaving a nickel sulfate solution. This solution was evaporated to dryness, and the residue analyzed 21.8 percent nickel, 0.05 percent iron, and 0.01 percent copper.
Waste-Plus-Waste Treatment Method
While the preceding procedures arc technically feasible, methods of reducing the cost of such treatments were continually sought. As the organic-cyanide (NB) rack-stripping waste is strongly alkaline, the addition of the nitric acid waste to the NB waste was considered an inexpensive first step to neutralize and concentrate both wastes before proceeding to recover the metal values. This procedure would be very dangerous in the order of addition were reversed (if NB waste were added to HNO3 waste) or if too much HNO3 waste were added to an NB waste with a high cyanide content, because in either case highly poisonous hydrogen cyanide (HCN) gas would be evolved. A nitric acid waste was added slowly with stirring to an NB waste until the pH of the solution was approximately 4.5. In a number of tests, this caused almost quantitative precipitation of all the metals as cyanides. During the neutralization, the evolved gas was collected in a 0.1 molar NaOH solution to determine if any HCN was produced. After neutralization, the NaOH solution was analyzed using an electrode sensitive to cyanide ions. No cyanides were detected, and the detection limit of the analytical method is 0.08 ppm of cyanides. This indicator, that essentially no HCN was evolved under the test conditions.
After the precipitated metal cyanides were filtered off, the filtrate also analyzed less than 0.08 ppm of cyanides. X-ray diffraction analysis, chemical analysis, and differential thermal analysis revealed that the precipitate was CuCN, Ni(CN)2·4H2O, and Cu2Fe(CN)6 · 7H2O. The mixed cyanide precipitate analyzed 24.0 percent nickel, 23.6 percent copper, and 0.9 percent iron.
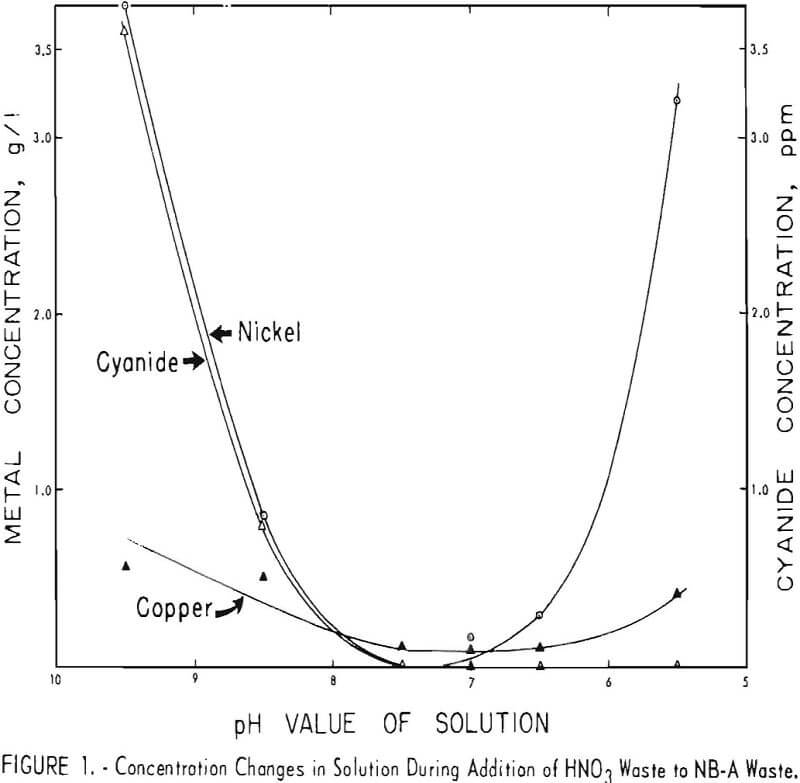
The changes in the concentrations of copper, nickel, and cyanide ions during the addition of HNO3 waste to NB-A waste are shown in figure 1. As the pH of the solution decreased, the concentration of each ion decreased to a very low value. Further additions of HNO3, waste introduced metal ions that were not precipitated and excess acid. However, the evolution of HCN was still negligible, apparently because all of tho cyanides were precipitated. Figure 1 shows that pH values of 7 or 7.5 were optimum for the precipitation of copper, nickel, and cyanides from these wastes.
Nickel and copper products were recovered from the mixed cyanide precipitate by a few additional steps. The precipitate was heated in air at 250° C to convert the metal cyanides to oxides, which were identified by X-ray diffraction as NiO, CuO, and FeO. When heated in air, the mixed cyanides ignite and undergo a self-sustained oxidation reaction, during which local temperatures may rise far above the furnace temperature. The mixed metal oxides were digested for 2 hours at 100° C in 10 percent sulfuric acid, which converted about 95 percent of the oxides to sulfates. The sulfates were dissolved in water, ferric hydroxide was precipitated with lime, and copper was recovered by controlled potential electrolysis as described before. The recovered copper contained less than 0.01 percent iron and less than 0.01 percent nickel.
The remaining nickel sulfate solution contained 0.02 percent copper and 0.02 percent iron, and probably could be recycled directly to nickel plating baths.
Other Waste-Plus-Waste Combinations
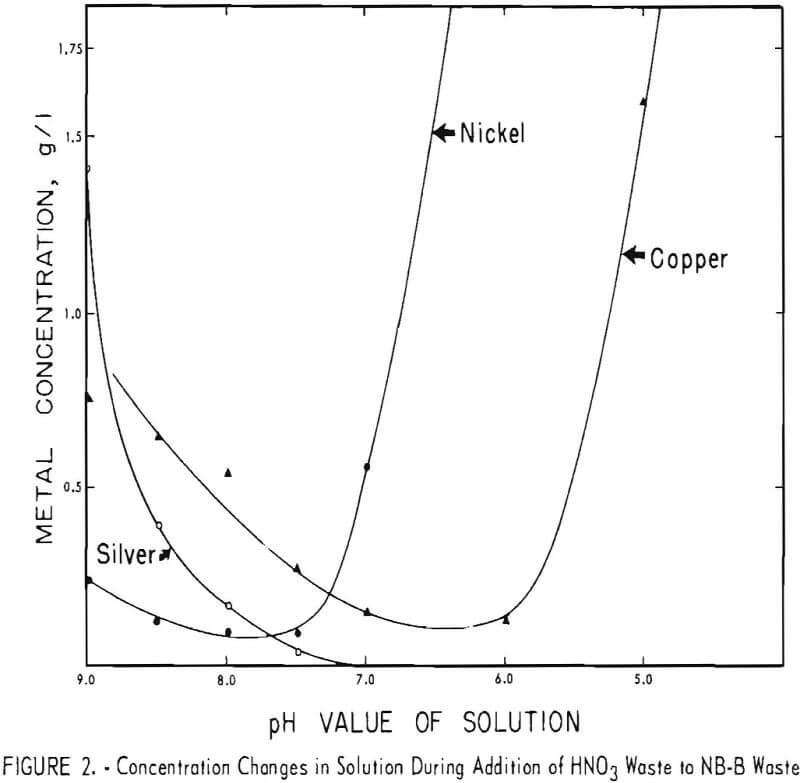
Because the results of these tests appeared to be promising, both technically and economically, additional tests were made on other waste-plus-waste combinations to determine if the method could be successfully applied to a wide variety of acid and alkaline wastes. Four other combinations of the wastes listed in table I were investigated. The results are shown in figures 2 through 5. It can be seen that the optimum pH values of the waste-plus-waste mixtures (the pH values where the maximum amount of metals and cyanides are precipitated) varied considerably with different waste combinations, ranging from about 5.5 to 7.5.
The optimum pH values appear to be 7 or 7.5 in figure 2, 6 in figures 3 and 5, and 5.5 or 6 in figure 4. Cyanide concentrations were below the detection limit throughout figure 2 and are not shown. In general, metal and cyanide precipitation was almost quantitative at the optimum pH values. Chromium
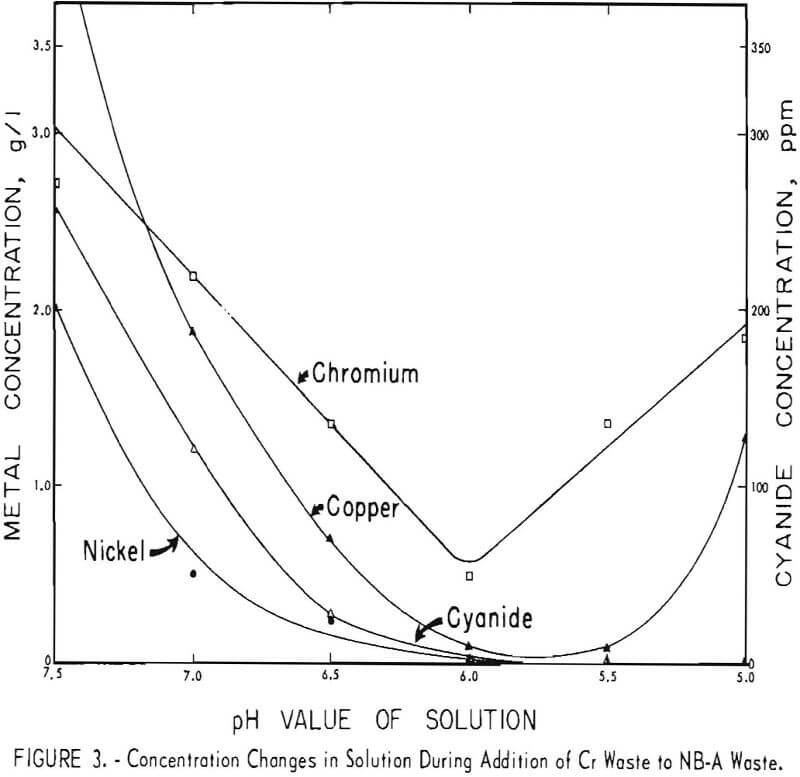
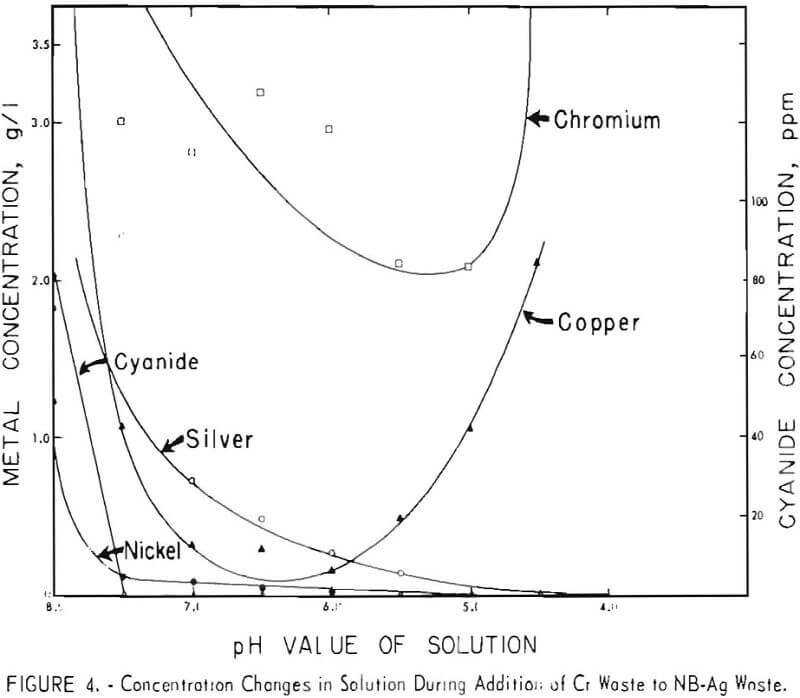
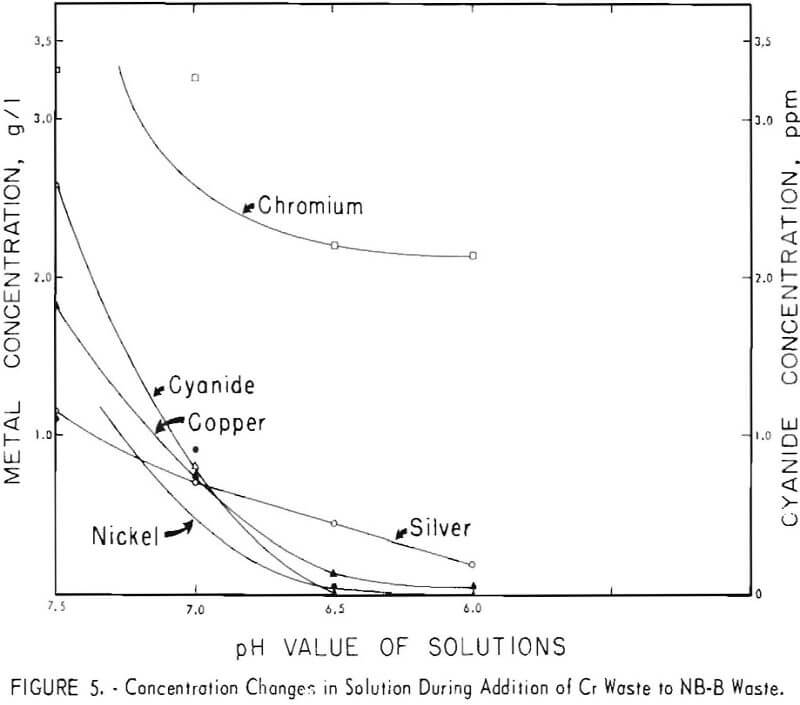
appears to be an exception in figures 4 and 5; however, 96 percent of the original chromium was precipitated in these two cases.
