Table of Contents
As part of its mission to reduce the environmental impact of minerals processing, the U.S. Bureau of Mines (USBM) investigated the characterization and treatment of Hg-contaminated wastes from an electrical-parts manufacturing plant. A literature search showed various hydrometallurgical methods for recovering Hg have been investigated on a laboratory scale, but there has never been a reported commercial application of any process other than thermal distillation for recovering Hg from ores and Hg-containing waste materials. Mercury has been known and used for 2,300 years in the history of mankind. Since Hg is not found in the elemental form in nature, the earliest Hg metal was probably obtained by smelting cinnabar (HgS) ores.
Status of Current Technology
Thermal distillation of Hg from Hg-containing waste materials, as well as HgS ores, has been reported in use in Japan, Norway, Sweden, the former Soviet Union, Germany, and Spain, as well as the United States. The wide acceptance of thermal distillation of Hg is based on its low boiling point (367 °C), the ease with which most Hg compounds are decomposed by thermal energy to form metallic Hg, and the low heat capacity of most Hg-containing waste materials and ores. Condensation of Hg vapor at ambient temperature is used to recover the Hg in a nearly pure state suitable for most uses. Only minimal energy is required to heat most Hg- containing materials to the temperature needed to decompose Hg compounds and to vaporize metallic Hg. The energy required to heat most Hg ores to recover Hg is generally less than the energy required to dry the same ore containing 15 pct nascent moisture. Condensation of Hg vapor from thermal distillation can recover up to 99.995 pct of the Hg contained in the distillate if no other gases are generated with the Hg vapor. Of course, vaporization of H2O and other gases decrease the efficiency of condensation in proportion to the amount of other gases mixed with the Hg vapor.
Thermal distillation of Hg ores has always had a reputation as an economical process. In fact, there are no known competing processes for the commercial recovery of Hg from ores. Historically, unbeneficiated Hg ores containing as little as 3 or 4 kg of Hg per metric ton of ore have been processed by thermal distillation solely for the value of the Hg recovered. For the past 80 years, the inflation-adjusted price of Hg has varied from a low of about $9/kg up to >$35/kg in periods of scarcity. The average inflation-adjusted price of Hg metal from 1910 to 1957 was about $22/kg. Thus, most Hg producers have remained economically viable by processing Hg ore at inflation-adjusted costs of <$55/mt of ore processed. The current Hg prices of $2 to $5/kg reflect surpluses and vastly increased supplies of cheap, recycled Hg. In addition to the thermal processing of the ore, the Hg processor also had to include costs of mining and crushing of the raw ore.
USBM Research
The research performed on the treatment of Hg- contaminated waste was part of an interagency agreement between the U.S. Environmental Protection Agency (EPA) and USBM to conduct treatability studies on certain wastes from metals-contaminated sites. Prior to the treatment studies at USBM Rolla Research Center (RORC), preliminary technological screening studies were conducted on the electrical wastes by the USBM Reno Research Center (RERC). These preliminary studies ruled out the feasibility of physical beneficiation methods such as sizing, gravity separation, and magnetic separation. Also, most leaching methods using acids, Cl-based solvents including Cl-O pressure methods, cyanides, and cupric chloride were ineffective in removing substantial amounts of Hg from the waste materials. Generally, Hg was only leached from the wastes when accompanied by some measure of decomposition or dissolution of the base materials. Hot, concentrated nitric acid, which completely decomposed the plastics in the waste material, was effective in removing Hg by dissolution.
The research at RORC was directed toward characterization of the electrical wastes and investigation of the factors that controlled the removal of Hg. The relative merits of acid-leaching processes, thermal desorption, and vacuum-thermal-desorption processes for Hg removal were also studied.
Characterization
The waste samples consisted of a mixture of electrical manufacturing parts and soil. About 50 pct of the sample consisted of Bakelite phenolic resin plastics, a phenolformaldehyde thermosetting resin used with wood or clay filler for manufacturing switch bodies, receptacles, plugs, and other electrical parts. Small amounts of miscellaneous materials such as magnetic and nonmagnetic metal contact plates, small wire fragments, screws, fluorescent starter cans, ceramic and glass fragments, and decomposed paper insulating material were also present in the waste. The soil that made up the remainder of the waste sample varied from large clayey lumps to sand and pebbles.
The samples obtained from the site were split by coning and quartering and then recomposed in order to obtain a measure of the dispersion of Hg in the wastes. Although some suspected Hg droplets were observed during these operations, subsequent microscopic examination failed to identify any elemental Hg at any magnification. Usually, the appearance of Hg resulted from small fragments of glass. To test the ability to visually detect Hg droplets in the samples, small Hg droplets, representing 1 ppm Hg, were spiked in the waste samples. Using similar operations, the presence of the Hg droplets was readily detectable.
The individual sample analyses and composite analyses showed an average Hg concentration of 396 ppm. The standard deviation of 15 waste samples was 30 ppm. The distribution of Hg in the waste samples was surprisingly uniform and homogeneous between the samples. Table 1 shows sample analyses from the three USBM laboratories used. Although the appearance of the wastes would suggest that they would be more likely to be distinctly non- homogeneous, some other factor was needed to explain the consistency of the analyses.
At some point in the life of these wastes, they had been decidedly nonhomogeneous. Mercury canisters, which are part of silent switches found in the wastes, typically contain 8 to 10 g of liquid Hg. The number of Hg canisters found in the samples indicated that the Hg content of the waste could account for only about 1 pct of the total Hg originally present in the opened and discarded canisters.
Although no information is available on the disposition of the Hg in the canisters, it is probable that Hg recovery was undertaken before disposal of the canisters. The lack of evidence of liquid Hg in the waste indicates that the Hg that was originally liquid was extremely finely dispersed, incorporated into other materials by adsorption, or simply vaporized. Most likely, a combination of factors controlled the fate of Hg in the waste. Mercury has a relatively high vapor pressure at ambient temperatures that could easily account for the disappearance of liquid Hg over a period of many years, with or without losses of Hg vapor to the atmosphere. Many substances containing carbonaceous materials are known to be efficient absorbers of Hg vapor.
Additional physical characterization of the wastes further supported the absence of elemental Hg in a form that could be easily separated by physical-, gravity-, or chemical-liberation methods.
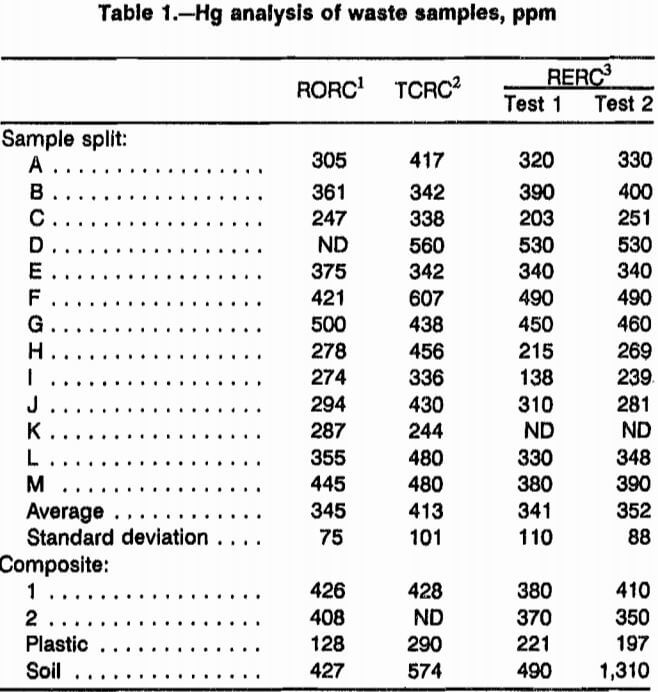
Note.—Average of composite analyses was 396 ppm and standard deviation of composite was 30 ppm.
Test 1
Samples of the Bakelite phenolic resin plastics found in the waste sample were separated from the ground composite samples by flotation methods. The plastics sample was further purified by repeated grinding and size classification using a mortar and pestle. This removed most of the external surface area of the plastics. The more friable impurities and surface layers of the plastic were removed by screening and washing, leaving a sample of internal plastics. The residual Hg content of the internal plastics averaged 209 ppm, or about one-half of the concentration of Hg in the bulk samples.
Test 2
The Hg-vapor concentration was measured in the closed bags containing the bulk waste samples. The maximum observed Hg-vapor concentration was <0.07 mg/m³. The true vapor pressure of Hg at 20 °C (10.9 mg/m³) is over 100 times greater than the observed vapor concentration in the equilibrated waste samples. The chemical potential and vapor pressure of adsorbed Hg is less than the vapor pressure of pure Hg.
Test 3
The Hg-vapor concentration of the waste samples increased very little during fine grinding with a mortar and pestle. This indicates the absence of free droplets of Hg metal that rapidly vaporize as the surface oxide layer is disturbed.
Test 4
The Hg-vapor concentration measured during grinding of a foundry sand sample spiked with a few parts per million of liquid Hg that was nearly equal to the theoretical vapor pressure of Hg. Adsorption isotherms for many substances are more linear than the corresponding equilibrium vapor pressures for the same pure substances.
Test 5
Drying of the waste samples at 100 °C did not produce significantly higher vapor concentrations of Hg. The true vapor pressure of liquid Hg approximately doubles for each 10 °C increase in temperature, going from 10.8 mg/m³ at 20 °C to 2,520 mg/m³ at 100 °C.
Test 6
Foundry sand (composed of fine quartz sand, with 10 pct bentonite clay, and a small amount of carbon), when spiked with small amounts of Hg and finely ground, showed high initial Hg-vapor concentrations that decreased by 90 pct within 30 days. After 4 months, the vapor concentrations were <0.005 mg/m³. Mercury vapor is known to be readily adsorbed by most semiporous and carbonaceous materials.
Test 7
Extensive leaching tests with H2SO4 and HCl at elevated temperatures and acid concentrations ranging from 10 pct up to concentrated acid removed only minute amounts of Hg from the waste samples. The corresponding fractional weight losses of the leached samples tended to be equal or greater than the fractional Hg removal, indicating that the host plastics dissolved before the Hg.
Test 8
The ground waste samples passed the EPA toxicity characteristic-leaching-procedure test for hazardous substances with only trace leaching of Hg in the acetic acid medium.
The consensus of these characterization tests lends strong support to the presence of Hg in the waste samples as a tightly adsorbed species. The adsorption of the Hg by the humus content of the soil, the decayed paper and wood materials, and the wood-flour-filled Bakelite phenolic resin plastics was undoubtedly assisted by extended exposure to Hg vapor in the waste dump over long periods of time. From available information, the wastes may have been in the waste dump for over 20 years, which would have been adequate time for small quantities of liquid Hg droplets to vaporize and disperse by diffusion within the waste materials.
Mercury-Vapor Sorption
The phenomenon of Hg-vapor sorption is well recognized and accepted. Perhaps the concept of metallic- vapor sorption is not readily apparent because Hg is the only metal capable of producing substantial vapor concentrations at ambient temperatures below the decomposition temperatures of most efficient adsorbing materials. However, vapor sorption of many vapors is a well-recognized physical principle. Gaseous materials such as Hg vapor diffuse into a solid host material because of differences in concentration. This driving force for adsorption continues until equilibrium conditions are established between the vapor phase and the solid phase. The solubility of one material in another is generally governed by the rule “like dissolves like.” Mercury vapor exists as a nonpolar molecule that would likely be most soluble in nonpolar substances such as other metals and nonpolar hydrocarbons.
Physical adsorption is another type of sorption. This process differs from dissolution and absorption because it is more of a surface process and adsorbed substances are held by physical forces that are not understood as well. Sometimes, molecular-sized pores serve as molecular traps, and in other cases, weak electrostatic forces operate at the surface. Adsorption processes are the basis for the application of gas-solid chromatography, which is often used to separate many fixed gases. Various materials have been shown to adsorb Hg vapor by either physical or chemical solubility processes. A wide range of Hg-vapor adsorption has been observed for clay-like minerals and organic materials.
The adsorption of Hg vapor increases with Hg-vapor concentration. The adsorption behavior of Hg on various materials can be described by the Freundlich adsorption isotherm, which relates the vapor pressure of the adsorbed species to vapor concentration.
The Freundlich adsorption isotherm is defined as
x/m = kCn…………………………………………………………………………(1)
where x = = amount of Hg vapor adsorbed in micrograms,
m = mass of the adsorbent in g,
C = concentration of Hg vapor in µg/m³,
and n and k = experimentally determined constants.
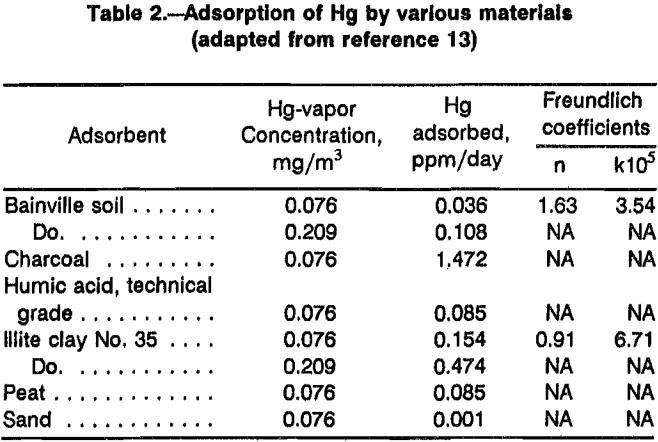
Table 2 shows the adsorption characteristics of various soil-like materials and organic materials for Hg vapor and the experimentally derived Freundlich adsorption coefficients. Materials containing organic matter and pure organic materials adsorb Hg vapor more rapidly than clay-like and mineral materials. Charcoal adsorbed about 20 times more Hg vapor than peat or humic acid, and peat adsorbed twice as much Hg as the soil from Bainville, MT. It is apparent that the nature of the organic matter, as well as the humic acid content of the soil, are important factors governing the adsorption of Hg vapor.
The ultimate adsorption capacity achieved by cumulative exposure to Hg vapor until equilibrium conditions prevail also varies for different materials. Adsorption saturation can be achieved in much less time with Bainville soil than with organic materials such as peat. This is probably an indication that the total adsorption of Hg vapor is more dependent on internal solution with organic materials than with mineral materials that make up the bulk of soil. The Freundlich adsorption isotherms show that proportionately greater Hg adsorption takes place as the vapor concentration of Hg increases. Peat-like materials exposed to Hg vapor would accumulate about 300 ppm Hg in as little as 20 days if exposed to Hg-vapor concentrations of 21.4 mg/m³ equal to the Hg-vapor pressure at 30 °C.
The adsorption process for Hg on soil transforms the Hg to a much less volatile adsorbed form in a tightly bound matrix. Experiments using radioactive tracers adsorbed on dry soils have shown that Hg adsorption is nearly an irreversible process. No detectable Hg could be removed from the dry soil by application of a vacuum for up to 24 h, heating to 110 °C for up to 2 h, or exposing the soil to a stream of air.
Thermal Desorption
Thermal desorption is the reversal of the adsorption process by addition of heat. The critical factors affecting the efficiency of the thermal desorption of Hg are the vapor pressure of the adsorbed Hg, and mass transport in the vapor phase. The Freundlich adsorption isotherm relates the vapor pressure of the adsorbed Hg to the concentration of the adsorbed species in the adsorbent. The Freundlich isotherms for most adsorbed species, including Hg, are much lower than the vapor pressure of the pure substance at low temperatures. At low temperatures and low vapor pressure, the Freundlich isotherm usually shows a linear relationship between vapor pressure and adsorbed concentration. However, as adsorbed concentrations approach the solubility limits or adsorption capacity of the adsorbent, the slope of the isotherm decreases rapidly. The Freundlich isotherms typically decrease rapidly with increased temperature and finally become almost single-valued at or above the boiling point of the adsorbed substance as adsorption capacity is greatly reduced. These characteristics of the Freundlich adsorption isotherm are very useful for an adsorbed substance such as Hg, which has a low boiling point of 376 °C. It means the adsorbed Hg can be desorbed easily and completely from the adsorbent by heating. This is the foundation for a clean separation process that separates the adsorbed Hg in the form of a concentrated vapor phase that is easily removed from the solid adsorbent phase.
Thermal desorption of Hg at temperatures above the boiling point of Hg should theoretically increase the rate of desorption by approximately as much as the difference between the vapor pressure of Hg at high temperatures and the Hg-vapor pressure of the adsorbed Hg. Since the vapor pressure of adsorbed Hg is near 10 -5 atm at room temperature, it is possible to increase the desorption rate by as much as 10 5 by heating the adsorbent. This shows more clearly how reduction of the pressure using a vacuum has a much smaller effect on the desorption rate than temperature.
Once desorbed, the Hg vapor must be removed from the reactor to maintain the driving force for the process. The driving force is the differential vapor pressure provided by the thermal-desorption equilibrium isotherms. The conditions of vapor-solid equilibrium also mean readsorption of Hg vapor can take place as vapor concentration increases or as the temperature decreases. Purge-gas flow is necessary to physically remove desorbed Hg and to greatly increase the efficiency of Hg removal. The purge-gas flow reduces the Hg-vapor concentration in contact with the adsorbent, maintains a large driving force for thermal desorption, and prevents readsorption of the Hg vapor.
Although it is possible in principle to achieve the same process objectives with vacuum operation as it is with purge gases, the practical aspects of vacuum operation are considerably more difficult. Purge gas at atmospheric density removes Hg vapor in nearly ideal plug-flow patterns with only minimal back diffusion flow because of the low diffusivity of Hg in the high-density purge gas. Vacuum removal of Hg vapor is dependent solely on diffusion of the Hg vapor by random molecular motion. Even with a relatively high vacuum, significant amounts of Hg vapor remain in contact with the adsorbent and diminish the driving force for thermal desorption; whereas in contrast, the concentration of the purge-gas phase has no theoretical effect on the driving forces required for thermal desorption, which depends only on the partial pressure of Hg in the purge gas.
Laboratory Thermal-Desorption Tests
Since most other physical beneficiation and chemical-based processes were ruled out because of the homogeneous distribution of adsorbed Hg, thermal desorption of Hg from the waste was selected for tests. Thermal-desorption tests, both on a laboratory retort scale and with a small rotary kiln, were conducted to define the operating parameters necessary to achieve Hg removal and recovery from the waste samples.
Laboratory thermal-desorption tests on the waste material were performed on 150-g samples of the material as received. Because of the heterogenous nature of the wastes in the samples, including bulky materials as well as thinner materials, it was likely the samples would behave in variable ways to the conditions of the desorption tests. The laboratory tests were made using an electrically heated retort. The retort was a 10-cm-diam, stainless steel pipe with thermocouple temperature monitors. Exhaust gases from the retort were removed by a flow of air that transported the Hg and other volatile material through a 1-cm-diam seamless, stainless steel condenser with water- jacket cooling. The condensate was collected in a receiver and the noncondensable exhaust gases passed through a canister of activated charcoal before being vented to the atmosphere.
The laboratory retort tests were used to determine the effects of temperature and heating time on the thermal desorption of Hg from the waste. Most of the initial tests conducted at temperatures >350 °C with purge airflow showed residual Hg levels of < 1 ppm with heating times of > 4 h. Longer heating times did not produce a significant further reduction in the level of residual Hg. Since some tests using low flows of purge air (200 cm³/min) through the reactor caused significant increases in the residual Hg content of the wastes, higher flow rates were generally used to eliminate the effects of airflow in thermal-desorption tests. Lowering the temperature of thermal desorption below 350 °C with a 2-h heating time caused the residual Hg levels to increase to >16 ppm. Results of characteristic-thermal-desorption tests are shown in table 3. Vacuum retorting of the wastes showed no detectable improvement in thermal-desorption rates. Neither heating time nor temperature requirements were reduced significantly. Airflow through the retort was a more effective means of removing the desorbed Hg vapor than vacuum conditions.
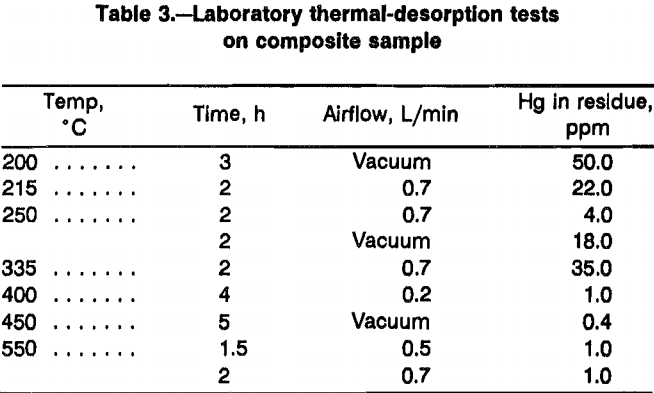
Excess air in contact with the waste at temperatures above 400 °C caused burning of the plastic and other organic material in the waste samples. Complete ashing of the plastics happened when the retort temperatures exceeded 500 °C, although the combustion proceeded slowly because of the limited amount of air available. Considerable additional heat was generated by the combustion of the waste plastics. Observation of the temperatures in the retort showed that the effect of the exothermic combustion reactions actually started at about 250 °C.
Large amounts of organic materials were distilled from the waste samples during the heating process. The semi-solid tarry condensate was evident at temperatures as low as 250 °C. The organic condensate had a strong phenolic odor from the decomposition of the wood-flour-filled Bakelite phenolic resin plastics, which contain phenol- formaldehyde resins. No condensed liquid Hg was found in the condensate. The aqueous fraction of the condensate contained <10 pct of the Hg in the organic fraction of the condensate. Although the recovery of Hg metal was low for the laboratory tests, monitoring of the exhaust gases from the activated-carbon adsorbent canister showed <0.002 mg/m³ in the exhaust gases.
Rotary-Kiln Thermal-Desorption Tests
A 10-cm-diam by 76-cm-long rotary kiln was used to conduct thermal-desorption tests on batch samples of up to 600 g of the wastes. The primary differences between the scaled-up rotary-kiln system and the laboratory retort used tests were the tumbling of the sample and the addition of an afterburner apparatus to destroy volatile organic materials. The rotary kiln was fitted with rotary seals fabricated from graphite cord packing. These sealing techniques provide a leakproof closure and Hg-vapor concentrations in the air surrounding the seals contained <0.002 mg Hg/m³, the detection limits for the Hg detector. The afterburner was a 3-cm-diam tube furnace with ceramic packing that ensured complete combustion of the exhaust gases generated in the rotary kiln. The after-burner was operated at 1,000 °C. Like the laboratory retort, the exhaust gases were cooled, condensed, and passed through a bed of activated carbon to completely remove Hg vapor.
At temperatures above 450 °C with a heating time of 4 h, the residual Hg levels of the waste samples were reduced to < 16 ppm. At this temperature with an airflow rate of 4.5 L/min, the wastes were almost completely ashed. The weight reduction was 60 to 70 pct. Volume reduction was near 70 pct. A summary of results is shown in table 4.
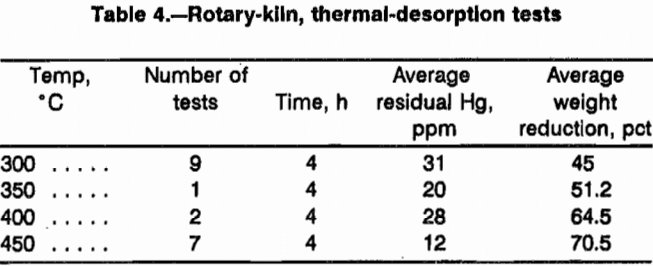
Between 33 and 38 pct of the waste sample weight was recovered as condensed H2O. The condensed H2O consisted of both nascent H2O in the wastes and the combustion product of organic materials burned in the after-burner. About 9.4 pct of the waste sample was nascent H2O. Although no facilities to perform organic analyses were available, no observable organic contamination was found in the clear, colorless condensate.
A Hg material balance failed to show more than 35-pct Hg recovery from the rotary-kiln, thermal-desorption tests. The remaining Hg was most likely entrained in the large volume of water condensate as fine droplets and not recovered by simple gravity separation. It may have been that the small amounts of desorbed Hg present failed to achieve physical and chemical adsorption with materials in the apparatus and offgas transfer train.
Mercury-Vapor Recovery and Environmental Control
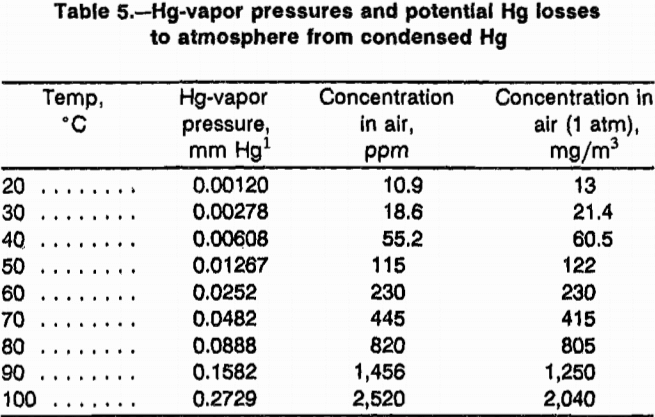
A major problem associated with thermal desorption of Hg from waste materials is removing the Hg from the noncondensable gases. The vapor pressure of Hg is much higher than the Occupational Safety and Health Administration (OSHA) limits for 8-h worker exposure to Hg in air. As shown in table 5, at the condensation temperature of 20 °C, the Hg-vapor pressure is 13.0 mg/m³, which is 260 times higher than the OSHA limit of 0.050 mg/m³.
There are a number of processes for removing Hg from gas streams often encountered in mineral processing.
Two processes seemed to be best adapted to remove Hg from the air purge stream used in thermal desorption of Hg from organic wastes, (1) activated-carbon scrubbers that remove Hg by sorption, and (2) liquid scrubbing with sodium hypochlorite solution. Hypochlorite leaching of HgS ores has been used to take Hg into solution as HgCl2. Continuous recycling of the leaching solution causes Hg concentrations to increase until all of the chloride is converted to HgCl2. At this point, continued addition of elemental Hg causes the reduction of HgCl2 to insoluble HgCl that can be removed by solids-separation methods.
Scrubbing of Hg vapors with sodium hypochlorite solution was shown to be ineffective for removing Hg from the kiln offgases. The cause was presumed to be the high acidity created by large amounts of CO2 in the rotary-kiln offgases. In the absence of viable methods to buffer the acidity, the hypochlorite scrubbing process was abandoned in favor of activated-charcoal adsorption. An activated-charcoal adsorber column 5 cm in diameter and 40 cm long was constructed to adsorb Hg from the kiln offgas stream and was used continuously during kiln operation.
Mercury-vapor emissions from the activated-carbon adsorber column were monitored at 15-min intervals during operation of the thermal desorption. The maximum Hg concentration measured in the exhaust gas was 0.005 mg Hg/m³ for only one 15-min operation period. At all other times, no detectable Hg emissions were measured at the detection limits of <0.002 mg Hg/m³.
Based on the initial Hg content of the waste samples of 396 ppm, <0.003 pct of the desorbed Hg was released to the atmosphere during the thermal-desorption tests performed on the waste samples. In addition, the use of the activated-charcoal adsorber maintained the Hg concentration of the exhaust gases at a level 20 to 100 times lower than the Hg-concentration limits required by OSHA regulations for 8-h workday exposure.
The sorption capacity of the activated-charcoal adsorber could not be determined without extremely long-term testing with Hg-saturated vapor. Various literature sources indicate that activated charcoal can adsorb 25- to 35-pct Hg vapor before the saturation or breakthrough concentrations are reached. Recovery of Hg from activated charcoal can be achieved by thermal desorption and condensation of Hg by the same process used for waste materials.
Summary and Conclusions
Discarded manufacturing wastes from an electrical-parts plant were characterized and treated by thermal desorption to remove and recover Hg contamination. Numerous characterization tests clearly demonstrated Hg was present as adsorbed Hg in the Bakelite phenolic resin plastics, organic material, and soil that made up most of the waste samples. No liquid Hg droplets from disposal of Hg-containing silent switches were visible in the waste. Other tests showed that the Hg concentration in the internal plastics was approximately one-half the concentration in the bulk sample and the very low vapor pressure of Hg in the samples indicated adsorbed Hg. Further tests showed that the Hg-vapor pressures were not increased by fine grinding or heating to 110 °C, and leaching of the waste material with H2SO4 and HCl removed only insignificant amounts of Hg.
Thermal-desorption tests on the waste samples were performed using both a laboratory-scale retort and a 10-cm rotary kiln, and showed the Hg content could be reduced to <1 ppm Hg from 396 ppm by heating to 350 °C for 4 h with purge airflow. Vacuum retorting was shown to have no advantage over use of purge air.
Although Hg recovery by condensation was difficult because of waste material decomposition, Hg-vapor emissions monitoring showed that <0.003 pct of desorbed Hg was lost to the atmosphere. The use of an afterburner on rotary-kiln tests destroyed most of the products organic material decomposition, and activated-carbon adsorber columns reduced Hg emission levels to < 1 pct of OSHA work-atmosphere standards and to less than 0.01 pct of the Hg-vapor pressure at room temperature.
It is presumed that because of high vapor pressure of Hg at room temperature and the tendency of many carbonaceous materials to adsorb Hg, many, if not most, Hg-contaminated wastes would contain substantial amounts of adsorbed Hg. Thermal retorting and thermal-desorption processing of Hg-containing materials is inherently a process requiring both low-capital and low-energy consumption that can produce, in most circumstances, a nearly pure Hg product by condensation.
Additional research would be required to demonstrate the effectiveness of the afterburner in the destruction of organic decomposition products and in improving the recovery of Hg from low-concentration waste materials.
