Table of Contents
The Bureau of Mines studied the extraction of lithium from lithium- containing clays by chlorination with hydrogen chloride (HCl). In bench-scale laboratory investigations, HCl-H2O mixtures were used to selectively chlorinate lithium, but not calcium or magnesium, in lithium-containing clays. The addition of calcium carbonate to the clay was found to improve the lithium recovery. Reaction conditions found to affect the lithium recovery were ratio of clay to carbonate, reaction temperature, and HCl concentration. The best conditions for selective chlorination of the lithium were 2:1 clay-carbonate, 750° C, and 20 wt-pct HCl. The experimental results and trends in lithium extraction are explained using thermodynamic relationships.
The Bureau of Mines has studied the extraction of lithium from nonconventional resources to meet its goal of maintaining an adequate minerals base for the Nation’s future economic and strategic needs. Lithium is expected to be in short supply as electrical storage batteries and fusion power are developed. This report covers the selective extraction of lithium-containing clays by chlorination with HCl.
Presently, lithium is almost exclusively obtained from two sources-(1) spodumene and similar minerals such as amblygonite and lepidolite and (2) lithium-containing brines found in Nevada and California. As the demand for lithium increases, these sources will be unable to supply all the Nation’s needs, and recovery of lithium from the nonconventional resources will become increasingly important.
Most of the lithium produced today comes from spodumene rained in the tin- spodumene district between North and South Carolina. A description of the known conventional lithium deposits in the United States is given by Norton and Schlegel. Significant amounts of lithium have recently been found in the clays of the McDermitt Caldera, which lies in northern Nevada and southern Oregon. A description of the mineralogy and distribution of these clays is given by Glanzman. If methods are found to extract the lithium from these clays, a major portion of this country’s lithium demand could be met by these reserves.
Industrial methods for the extraction of lithium from its ores have been discussed by Ellestad and Clarke. These methods include (1) base exchange with alkali sulfates, (2) processing based on roasting with lime, (3) miscellaneous processes based on roasting with combinations of CaCO3, Ca(OH)2, CaCl2, and CaSO4, and (4) roasting with sulfuric acid. The most important commercial process is the extraction of lithium by roasting with sulfuric acid. This process is described by Ellestad and Leute.
Lithium has also been extracted from its ores by chlorination. Many processes have been proposed whereby the ore is roasted with a chloride salt of potassium, sodium, or calcium. MacDougall has proposed a method to chlorinate lithium by mixing the ore with carbon and reacting with chlorine. The chlorination of lepidolite with hydrogen chloride has been investigated by Lof and Lewis. They found that lithium extraction increased with the chlorination temperature up to 950° C whereupon the lepidolite began to melt and the reaction rate decreased rapidly. Above 900° C a large part of the lithium chloride was recovered as a volatile product. Additions of water vapor or air to the hydrogen chloride gas resulted in decreased lithium extraction.
The Salt Lake City Research Center investigators studied two types of chlorination using hydrogen chloride: first, chlorination with anhydrous HCl, and second, selective chlorination with HCl-H2O mixtures. Anhydrous chlorination was done to test how effectively HCl could be used to extract lithium from the clay. Selective chlorination was investigated to see if the unnecessary chlorination of major contaminating elements such as magnesium and calcium could be eliminated.
Thermodynamics
Since the metallic cations are usually associated with silica in a clay material, chlorination of the clay will follow the general reaction
2M (silicate) + 2HCl = 2MCl + 2SiO2 + H2O………………………………(1)
where M is a metallic cation. The Gibbs free energies for the chlorination of the most stable silicates of potassium, sodium, lithium, calcium, and magnesium according to the above reaction at various temperatures are given in figure 1. If the chlorination follows this general reaction, water
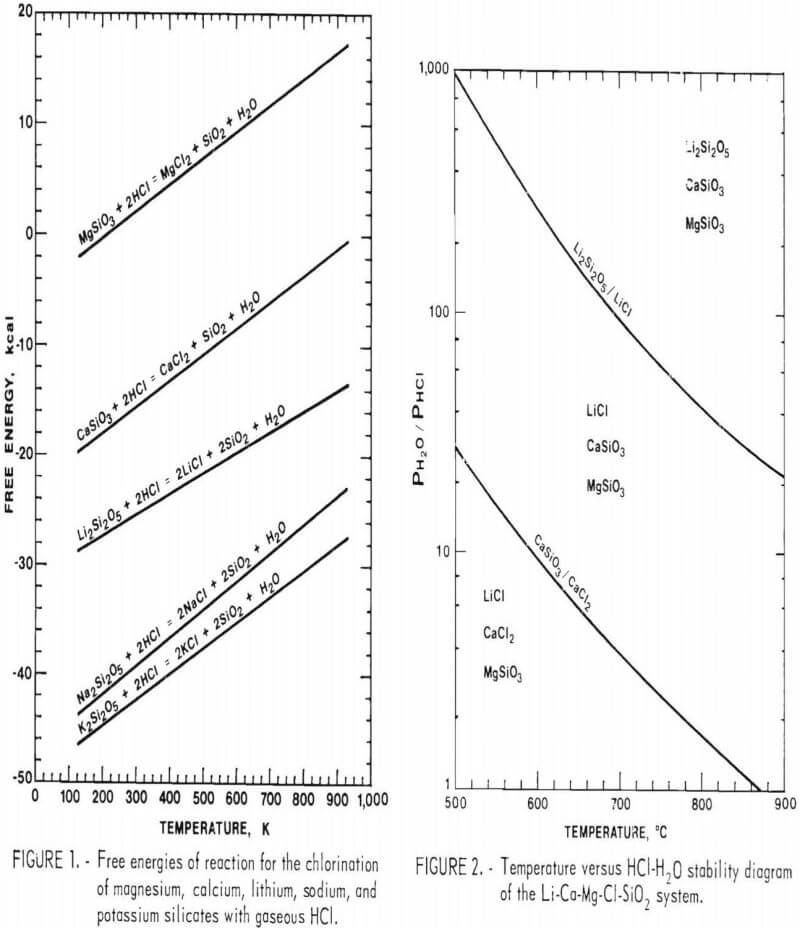
concentrations above the equilibrium value will reverse the chlorination. The ratio of water vapor to hydrogen chloride (H2O/HCl) that exists at equilibrium for the different silicates can be calculated from thermodynamic tables.
A temperature-composition phase diagram relating the equilibrium H2O-HCl ratio to the chloride-silicate phase boundary between 500° and 900° C is shown in figure 2. From this figure it is possible to predict the stable phase of each element at a given temperature and gas composition. For example, to avoid the chlorination of magnesium silicate and calcium silicate when using a H2O-HCl mole ratio of 8:1 (20 wt-pct HCl) the reaction temperature must be at a temperature above 615° C. If the H2O-HCl mole ratio is decreased to 4:1 (33 wt-pct HCl), the temperature must be increased to 700° C to avoid chlorination of magnesium or calcium silicates.
The net chlorination rate is related to the position of the gas composition-temperature point with respect to the chloride-silicate phase boundary. Above the boundary line the chlorination rate will be zero since the lithium silicate will be the stable phase. If the gas composition-temperature point lies on the boundary line, the net reaction rate will be zero since the silicate system is at equilibrium. Displacement of the gas composition temperature point to the left of the equilibrium boundary line results in the formation of lithium chloride, and the net chlorination rate increases because of the greater reaction driving force. Therefore at a given gas composition, lower temperatures could favor faster net chlorination rates, as long as the kinetic influence of decreased temperature remains unimportant.
The phase boundary between the chloride and the silicate depends on a function of the partial pressures of hydrogen chloride and water vapor determined by equation 2:
K = PH2O/PHCl²………………………………………………………(2)
where K is the equilibrium constant for reaction 1. Considering the chlorination of lithium and calcium silicates with hydrogen chloride at 1,000 K, figure 3 shows how the phase boundary changes with partial pressures of hydrogen chloride and water vapor.
This report focuses on the chlorination of lithium-containing clays by HCl for the recovery of lithium. The chlorination using anhydrous HCl and H2O-HCl mixtures will be discussed. The use of clay-CaCO3 mixtures to improve the selective chlorination of the lithium will be shown. Optimum conditions for the highest lithium recovery by selective chlorination of clay-CaCO3 mixtures will be given.
Materials, Equipment, and Test Procedures
Materials
The clay sample used in the HCl chlorination work was supplied by the Lithium Resource Appraisal Group of the U.S. Geological Survey and was obtained from the southwestern rim of the McDermitt Caldera. Mineralogical studies show that lithium is homogeneously distributed throughout the clay and it would not be practical to upgrade the lithium content by physical beneficiation techniques. Tests were therefore done using clay samples as received except for drying, grinding, and screening to minus 100- plus 200-mesh.
X-ray diffraction studies along with the clay’s chemical properties suggest that it is a montmorillonite-type of clay. The clay’s composition is given in table 1. Except for the silicon content the magnesium is the major cation, being 10 times more abundant than lithium on an equivalent basis.
Other materials used with the chlorination work were obtained as commercial reagents. Hydrogen chloride was used as either compressed gas or hydrochloric acid. Calcium carbonate and silicates of lithium, calcium, and magnesium were obtained from Pfaltz and Bauer as reagent-grade chemicals- The calcium, magnesium, and lithium silicates had compositions containing 16 pct Ca, 5 pct Mg, and 5 pct Li, Mixtures of clay and carbonate were first prepared by grinding the clay and calcium carbonate to minus 100- plus 200-mesh and then mixing them together by screening several times through a 100-mesh screen.
Equipment
Chlorination experiments were done in a 1-inch-diameter tube furnace using approximately 10 grams of sample contained in a ceramic boat. The furnace tube was Vycor glass. A schematic of the furnace setup is given in figure 4. The setup consists of a chlorinating agent supply system, the reaction zone, and a scrubbing system for volatile products. Anhydrous HCl and nitrogen were delivered to the furnace tube by metering their respective gases through gas flowmeters. Water-hydrogen chloride mixtures were obtained by containing a solution of hydrochloric acid in a flask and forcing the solution out through a flowmeter and into the furnace tube by pressuring the flask with nitrogen. The solution flowed down a capillary tube directly into the furnace hot zone.
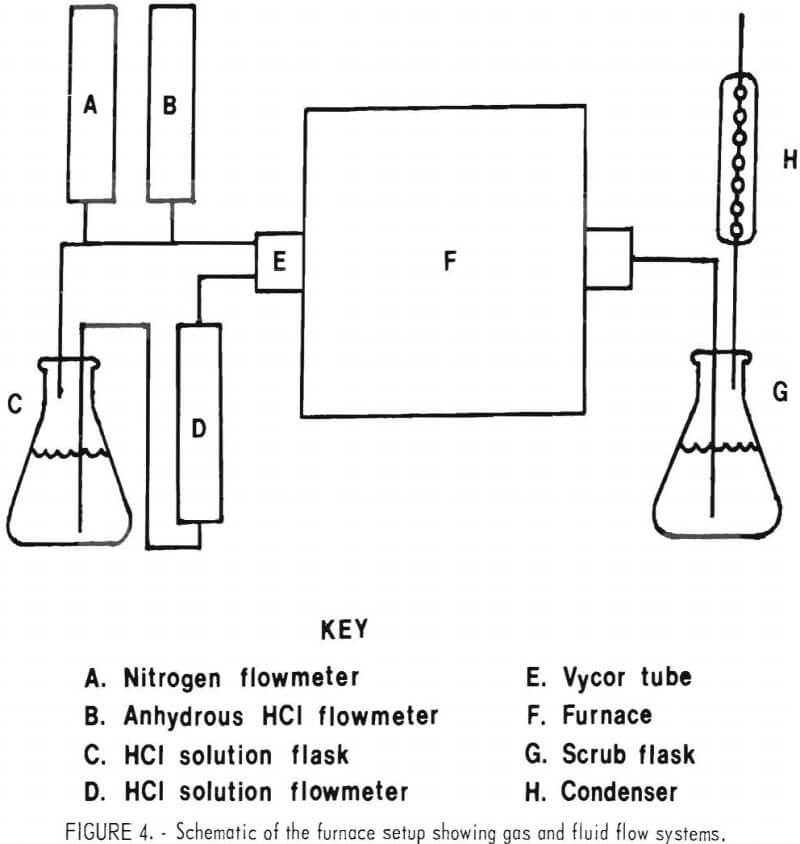
The furnace temperature was controlled by a Bristol on-off type controller. A thermocouple was placed on the outside of the furnace tube directly over the sample. Any gaseous products flowed through a water scrubber and condenser system to collect the volatile products.
Procedure
Samples were weighed into ceramic boats and placed in the furnace tube, then the system was purged with nitrogen. The furnace was heated under nitrogen to 700° C and held for 15 minutes to dehydrate the clay and any other materials being reacted. After dehydration, the samples were brought to the desired reaction temperature and the chlorination started. When the experiment was finished, the furnace was cooled under a nitrogen atmosphere.
After chlorination the calcined sample was water leached using 50 ml of of water at 80° C. The leach solution, residue, and any volatile condensate were analyzed, A mass balance was then computed between the input and output materials. When using the above procedure, a mass balance accounted for all elements within ±5 pct.
Test Results
Chlorination tests were divided into three general areas; First, general chlorination testing using anhydrous HCl; second, development of selective chlorination trends using H2O-HCl mixtures; and third, selective chlorination of clay-CaCO3 mixtures with H2O-HCl for the extraction of lithium from McDermitt clay. The extent of chlorination was measured as the amount of soluble chloride extracted from the sample after chlorination.
Chlorination With Anhydrous HCl
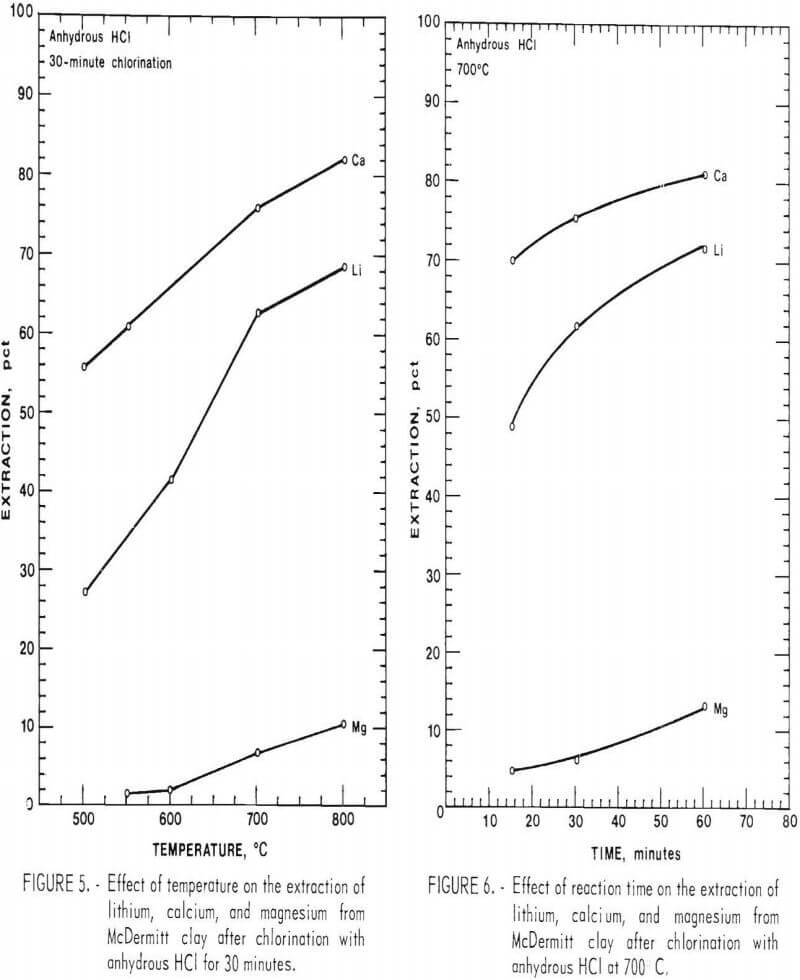
The general effects of chlorination on the extraction of lithium from clays were measured by chlorinating samples of McDermitt clay with anhydrous HCl. The effect of temperature on the extraction of lithium, calcium, and magnesium from McDermitt clay is shown in figure 5. The chlorination was run for 30 minutes using 70 cm³/min of anhydrous HCl. As the reaction temperature is raised, the extraction of all elements increases. However, the rate at which the extraction of lithium and calcium increases is greater than the rate at which the extraction of magnesium increases.
The low extraction of magnesium as compared to lithium can be explained by the difference in their free energies of chlorination. Figure 1 shows that the chlorination of lithium, potassium, sodium, and calcium all have negative free energies of chlorination. The chlorination of magnesium has a positive free energy of chlorination above 200° C, and therefore the extent of chlorination is slight because of the unfavorable thermodynamics.
The effect of reaction time on the extraction of lithium, calcium, and magnesium is shown in figure 6. Chlorination tests were run at reaction times of 15, 30, and 60 minutes using McDermitt clay, anhydrous HCl at 70 cm³/min, and a temperature of 700° C. Total extraction of each element increased with increasing reaction time, but the rate of extraction increased for magnesium while decreasing for lithium and calcium. (Rates of chlorination (extraction) are given by the slope of each curve at any point.) The increased extraction rate for magnesium is actually a function of the decreased rates of extraction for lithium and calcium, wherein more HCl is available to react with the magnesium and also the water vapor pressure is lowered, further increasing the extent of magnesium extraction. Thus, as the extraction of lithium becomes complete, high magnesium extraction will be obtained.
Selective Chlorination With HCl-H20 Mixtures
The high magnesium content of the McDermitt clay results in high consumption of HCl for its chlorination. Since HCl is an expensive reagent, selective chlorination of the alkali metals in preference to chlorination of magnesium is desirable.
Consideration of figure 2 shows that the proper HCl-H2O mixture will allow for selective chlorination between the different metal silicates. The trends predicted in figure 2 were verified by chlorinating various silicate mixtures using the predicted conditions of temperature and HCl concentration.
Initial experiments using mixtures of lithium, calcium, and magnesium silicates were run to test the selective chlorination conditions. Finely ground CaSiO3, MgSiO3, and Li2SiO3 were mixed in equal weights and chlorinated. The HCl concentration ranged from 8 to 33 wt-pct, and the reaction temperature ranged from 400° to 850° C. The conditions of chlorination used in these tests were chosen so that magnesium would not chlorinate while lithium would always chlorinate. Chlorination samples were water leached and analyzed for soluble chlorides. The extractions of magnesium, lithium, and calcium, given in table 2, correspond with the trends shown in figure 2. Magnesium extractions are not greater than 0.01 pct, and lithium extractions are always high. Variations in the chlorination conditions cause calcium to either chlorinate or not chlorinate. Comparison of the calcium extractions in tests 1 and 2 shows a major decrease at the higher temperature. The decrease in calcium extraction is consistent with the predicted chlorination trend. The increase in temperature from 550° to 700° C when using 20 wt-pct HCl crosses the phase boundary between CaCl2 and CaSiO3 at 615° C (as seen in figure 2), and this accounts for the decrease in calcium extraction with increased temperature. Comparison of tests 3 and 4 again shows a decrease in the calcium extraction with increased temperature. This decrease is also predictable based on figure 2. An increase in HCl concentration increases the temperature at which the phase boundary between CaCl3 and CaSiO3 occurs.
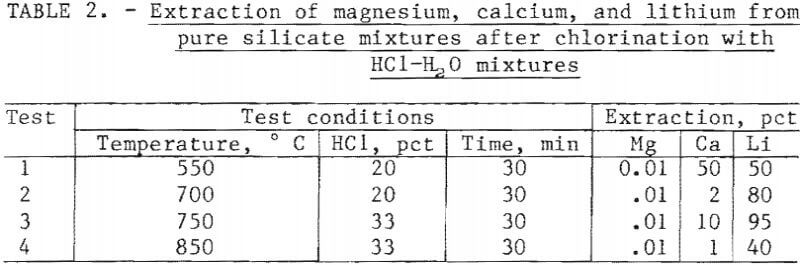
The results in table 2 show that the selective chlorination of lithium silicate is possible by using the conditions that can be predicted from figure 2. The selective chlorination is seen to depend on the reaction temperature and the HCl concentration. The use of water vapor in the chlorinating atmosphere did not limit the lithium recovery, and good extractions were obtained.
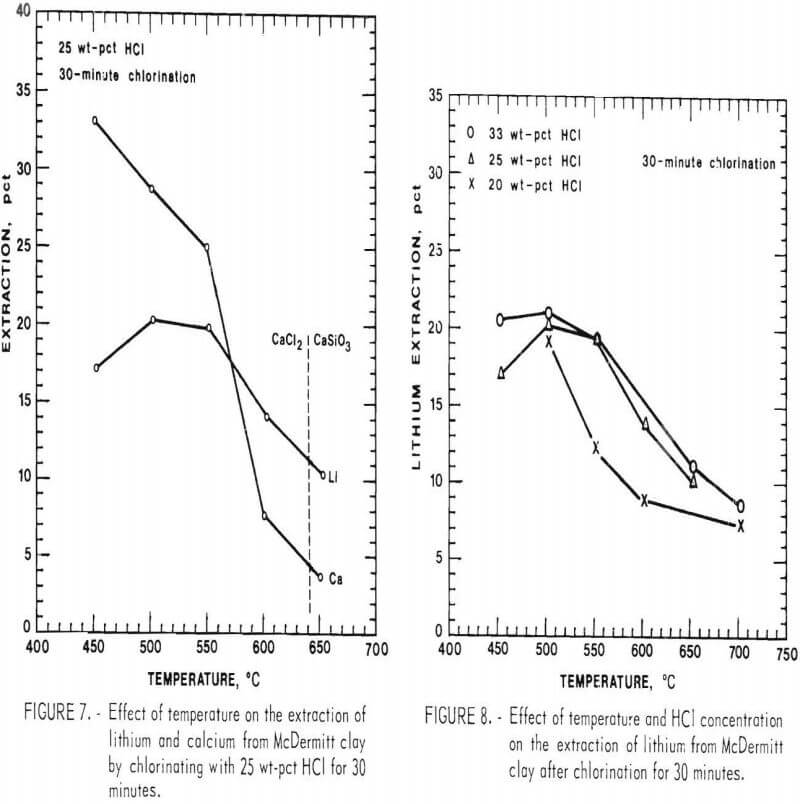
The selective chlorination of lithium in McDermitt clay was tried using H2O-HCl mixtures. A solution of 25 wt-pct HCl was used as the chlorinating gas, giving a 6:1 ratio of H2O to HCl. Samples were chlorinated at temperatures ranging from 450° to 700° C for 30 minutes and then water leached. Results are shown in figure 7. Superimposed on this figure is the CaCl2-CaSiO3 phase boundary when using 25 wt-pct HCl. The calcium extraction decreases rapidly once the chlorination conditions cross the phase boundary. Lithium extraction decreases slowly with increasing temperature; this occurs because the constant ratio of H2O to HCl results in less favorable equilibrium relationships at the increasing temperatures. Maximum lithium extraction was 20 pct at a temperature of 500° C.
Increasing the reaction time was attempted in an effort to increase the lithium extraction when using 25 wt-pct HCl. Samples of McDermitt clay were chlorinated for 60 and 120 minutes at 500° C, but lithium extraction remained at about 20 pct.
Increased HCl concentration was studied along with temperature variation. McDermitt clay was chlorinated for 30 minutes with HCl concentrations between 20 and 33 wt-pct and temperatures between 450° and 700° C. Results of these tests are shown in figure 8. Lithium extraction increased with temperature up to 500° C but decreased at higher temperatures. An increase in the HCl concentration gave only slightly better lithium extractions. The greatest lithium extraction was 22 pct at 500° C when using 33 wt-pct HCl, which is only 4 pct better than the extraction with 20 wt-pct HCl at the same temperature. Thus the selective extraction of lithium from McDermitt clay was limited to a maximum recovery of about 20 pct, and increases in the reaction temperature, HCl concentration, or time of reaction did not improve the lithium recovery.
Chemical analysis of the leached calcine showed large amounts of insoluble chloride. The insoluble chloride is assumed to be present as a chlorosilicate and to be formed by the reaction of the chloride with the free silica (SiO2 ) present in the clay. The chlorosilicate compound would have a structure similar to sodalite, Na8(AlSiO4 )6Cl2. The formation of a chlorosilicate would tie up the alkali metal chlorides so that these metals would not be water extractable. This could account for the low extractions of lithium.
The formation of the chlorosilicate might be avoided if the free silica in the clay was combined into a nonreactive compound. Thermodynamic calculations show that free silica should react with CaO at 700° C. If the chlorosilicate is tying up the lithium in an insoluble form, the addition of CaO as CaCO3 should improve the lithium recovery by decreasing the amount of free silica.
Based upon the analysis of free silica given in table 1, it is necessary to have a minimum clay-to-carbonate ratio of 4:1 to react with all the free silica. This ratio of clay-to-carbonate is based on the formation of CaSiO3 , which requires 1 mole of CaO per mole of SiO2.
Selective Chlorination of Clay-CaCO3 Mixtures With HCl-H2O
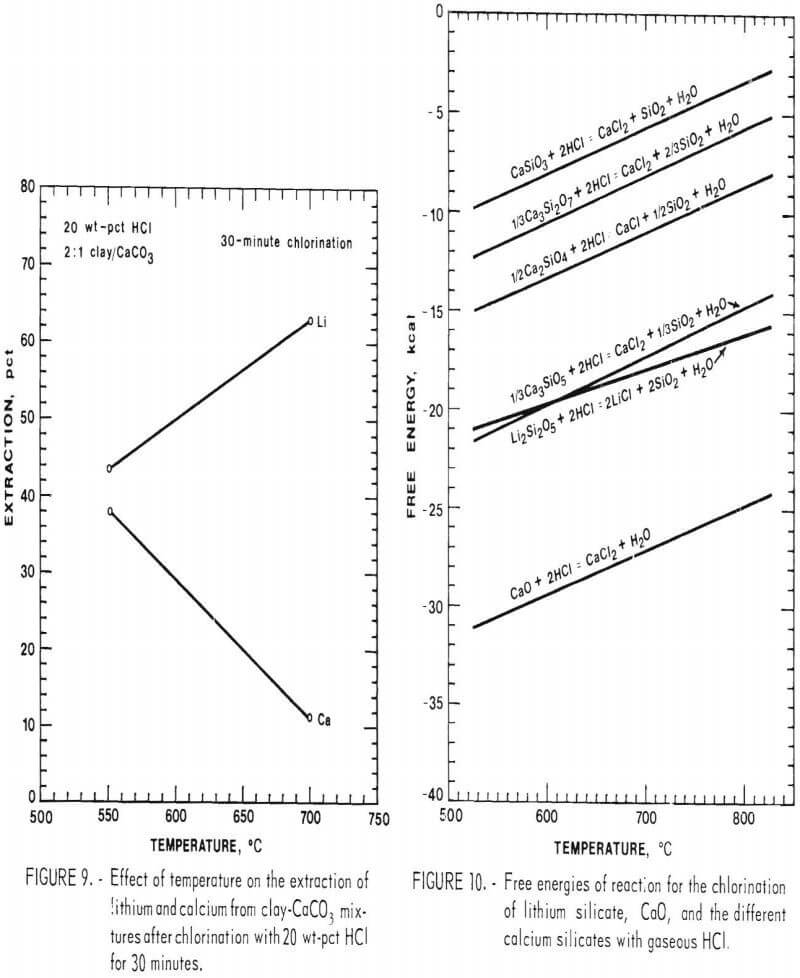
Initial experiments to evaluate the effect of CaCO3 addition on the lithium extraction were run using twice the amount of CaCO3 needed to tie up the free silica. Mixtures of McDermitt clay and CaCO3 with a 2:1 ratio were chlorinated for 30 minutes at temperatures from 550° to 700° C using 20 wt-pct HCl. Results for these tests are shown in figure 9. Lithium extractions increase from 43 pct at 550° C to 63 pct at 700° C, compared with a decrease in lithium extraction from 12 pct to 8 pct when chlorinated without the CaCO3 addition (shown in fig- 8).
Calcium extraction, because of the phase boundary between CaCl2 and CaSiO3 at about 640° C, decreases from 38 pct at 550° C to 11 pct at 700° C. The high calcium extraction at 550° C, seen in figure 9, is obtained because at this temperature the chlorination of calcium silicate is favored.
Several types of calcium silicates can be formed by the reaction of CaO and SiO2. The ratio of CaO to SiO2 will determine which of the different calcium silicates form. A clay-carbonate ratio of 4:1 corresponds to a 1:1 ratio of CaO to SiO2, which yields the silicate CaSiO3. As the CaO-SiO2 ratio is increased, the more basic silicates will be formed. Clay-carbonate ratios of 3:1, 2:1, and less give CaO-SiO2 ratios of 3:2, 2:1, and greater. CaO-SiO2 ratios of 3:2, 2:1, and 3:1 give silicates Ca3Si2O7, Ca2SiO4, and Ca3SiO5. CaO-SiO2 ratios higher than 3:1 form Ca3SiO5 plus CaO.
Free energy values for the chlorination of the various calcium silicates and CaO are compared with the free energy of chlorination for lithium silicate in figure 10. Selective chlorination between compounds with large differences in free energy is theoretically possible. Therefore, lithium silicate can be selectively chlorinated in mixtures containing CaSiO3, Ca3Si2O7, and Ca2SiO4. Clay-carbonate mixtures greater than 2:1 will form Ca3Si2O7, which has a free energy of chlorination about equal to that of lithium silicate. This makes selective chlorination between lithium silicate and Ca3SiO7 thermodynamically impossible. The free energy of chlorination of CaO is less than the free energy for the chlorination of lithium silicate, and therefore CaO will chlorinate in preference to lithium silicate. Based upon the above discussion, selective chlorination between lithium and calcium is then possible using clay-carbonate mixtures greater than 2:1. Clay-carbonate mixtures with less than a 2:1 ratio will have CaCl2 in the leach solution, because of the chlorination of Ca3SiO2 or residual CaO.
These investigations show that selective extraction of lithium in the McDermitt clay depends on the HCl-H2O ratio, reaction temperature, and the addition of CaCO3. A series of experiments investigated the effect of these variables on lithium extraction. The HCl concentrations used ranged from 8 to 20 wt-pct; reaction temperatures ranged from 600° to 750° C. The calcium
carbonate additions ranged from the minimum amount needed to tie up the free silica (4:1 clay-carbonate) to the maximum amount that would give a calcium silicate that could still be selectively chlorinated (2:1 clay-carbonate).
The effect of a change in chlorination conditions on the lithium extraction is shown in figure 11. The order in which a change in the chlorination conditions improves the lithium extraction is first, the amount of calcium carbonate addition; second, reaction temperature, and third, HCl concentration.
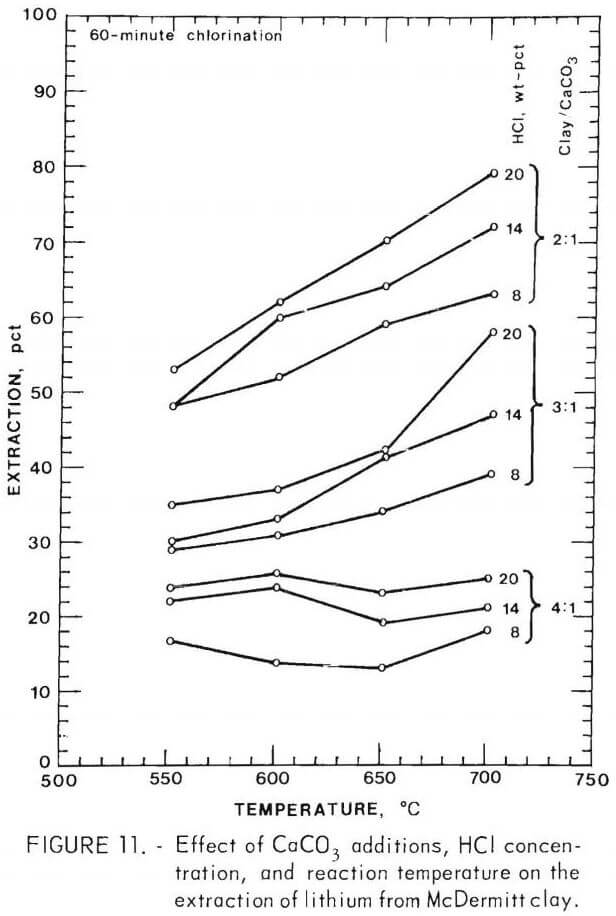
There is a 54-pct improvement in lithium extraction when the clay-CaCO3 ratio is decreased from 4-1 to 2:1 when using 20 wt-pct HCl at 750° C. Lithium extraction is improved 27 pct when the temperature is increased from 600° to 750° C when using a 2:1 clay-CaCO3 mixture and 20 wt-pct HCl. Lithium extraction is improved 19 pct when the HCl concentration is increased from 8 to 20 wt-pct when using a 3:1 clay-CaCO3 mixture and a temperature of 750° C.
There appears to be an interaction between the HCl concentration and the reaction temperature. Increasing either the reaction temperature or the HCl concentration improves the effect the other variable has on the lithium extraction. For example, when using a 3:1 clay-CaCO3 mixture at 600° C, an increase in the HCl concentration from 8 to 20 wt-pct increased the lithium extraction by 6 pct, but at 750° C an increase in HCl concentration from 8 to 20 wt-pct increased the lithium extraction by 19 pct.
The results given in figure 11 show that a 4:1 clay-CaCO3 mixture is the minimum amount of CaCO3 that will improve the lithium extraction. The optimum conditions for lithium recovery are 2:1 clay-CaCO3, 20 wt-pct HCl, and 750° C. Increasing any of these variables is impractical because clay-CaCO3 mixtures lower than 2:1 give a composition in which the calcium begins to chlorinate, temperatures above 750° C cause fusion of the powder that decreases the lithium extraction, and HCl concentrations higher than 20 wt-pct become expensive to obtain.
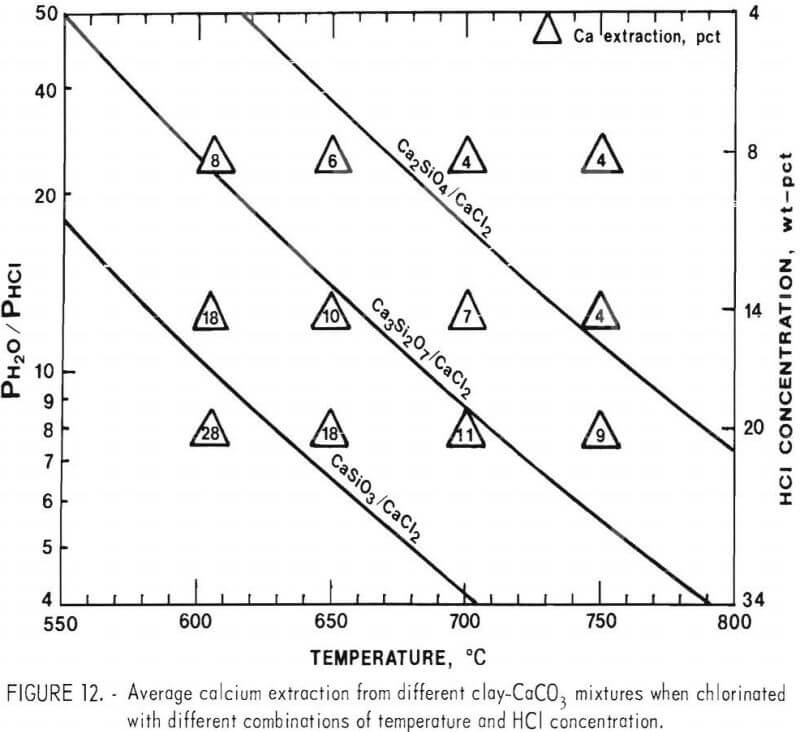
The amounts of calcium extracted in the above chlorination tests are listed in figure 12 at the different conditions of temperature and HCl concentration used for chlorination. Calcium extraction shown is an average from the three clay-CaCO3 mixtures.
Figure 12 shows a correlation between the average calcium extraction and the stability of the different calcium silicates. The average calcium extraction increases each time a phase boundary is crossed. This indicates that all three different calcium silicates are present in the calcine. The 4-pct calcium extraction to the right of the Ca2SiO4 phase boundary suggests that a small amount of either unreacted CaO or Ca3SiO5 is present. The high calcium extraction to the left of the CaSiO3 phase boundary is expected, since all the calcium silicates chlorinate under these conditions.
To minimize the extraction of calcium would require a temperature of 750° C and HCl concentrations less than 14 wt-pct. However, to optimize the lithium recovery requires 20 wt-pct HCl at 750° C. Therefore, the best conditions will be a compromise between the conditions that give the best lithium recovery and the conditions that give the minimum chloride loss to calcium chlorination.
Conclusions
The extraction of lithium from clays by chlorination with HCl was shown to be effective. Successful application of chlorination requires the selective chlorination of the alkali metals in preference to the other cations. Selective chlorination was possible with the use of HCl-H2O mixtures.
The selective extraction of lithium from various mixtures of pure silicates is shown. Chlorination of calcium and magnesium was restricted by using the proper combination of HCl-H2O mixture and temperature. Increasing the HCl concentration required an increase in the reaction temperature to maintain the selective extraction of lithium. The reaction conditions and trends were explained with the aid of thermodynamic relationships.
The selective chlorination of lithium in clays followed the predicted trends, but the maximum lithium extraction was limited by the high free silica content of the ore. The free silica appeared to react with the chlorides, forming an insoluble chlorosilicate that tied up the lithium and other alkali metals. The addition of CaO formed a calcium silicate with the free silica and allowed the lithium to be extracted more completely.
The order in which the reaction conditions improve the lithium extraction by selective chlorination was first, the amount of calcium carbonate addition; second, the reaction temperature; and third, the HCl concentration. A maximum lithium extraction of 80 pct was obtained using a 2:1 clay-calcium carbonate ratio, a temperature of 750° C, and 20 wt-pct HCl.
