Table of Contents
A major goal of the Bureau of Mines is resource conservation, which includes (1) maximizing mineral recovery from domestic resources and (2) recovering mineral values currently lost in scrap and waste materials. To help meet this goal, the Bureau of Mines has been investigating the recovery of various potentially valuable minerals from mineral- processing wastes.
One of the more promising areas of these studies is the recovery of barite from waste pond materials. Wharton determined the amount of barite contained in some Missouri waste ponds, and Brobst includes barite in old waste ponds in reserves data.
Barite is used in well-drilling muds, paint, glass, rubber, plastics, and barium chemical manufacture. The United States, while producing about 30 percent of world supply, still imports about 40 percent of its barite consumption. U.S. reserves of barite have historically been considered sufficient in the long term; however, Haines has suggested that U.S. reserves are not adequate to meet expected demand through the year 2000.
Domestic barite production increased from 1.1 million tons in 1974 to 1.7 million tons in 1978; however, during the same period, consumption increased from 1.7 to 2.8 million tons. The major factor in the increased barite consumption was the increase in oil- and gas-well drilling. Barite is an important ingredient in well-drilling fluids because of its high specific gravity, chemical inertness, and softness or non-abrasiveness.
In fiscal year 1979, the Bureau’s Tuscaloosa Research Center initiated a study to devise methods to recover barite previously lost in milling and to prevent such losses in the future. Flotation tests were conducted using waste materials from Georgia, Missouri, and Nevada. Results of these tests indicated that barite concentrates that met oil well drilling-mud specifications could be produced from these sources.
This report presents the results from additional flotation tests on four waste pond samples from past and present operations. The objective of these tests was to determine if a high percentage of the barite in the feed could be recovered while producing a product that met the minimum drilling-fluid specifications of a BaSO4 content of 92 percent and a specific gravity of 4.20.
Materials Procedures and Results
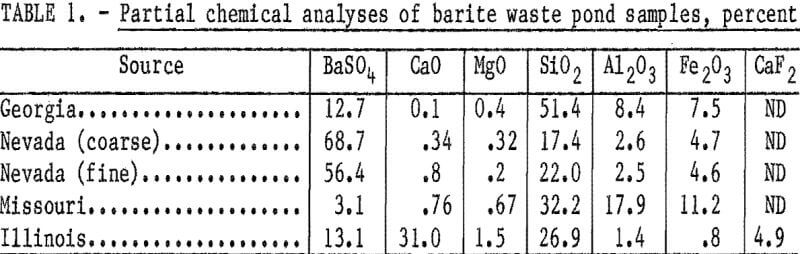
The character and particle size of the samples varied widely, and chemical analyses of the as-received samples determined that the barium sulfate (BaSO4) content ranged from 3.1 to 68.7 percent (table 1). Because of the inherent problems in obtaining representative samples from waste ponds, these samples cannot be considered to be representative of any significant tonnage of material. A tonnage estimate for any particular waste pond was not furnished by the cooperating barite producers; hence, these studies represent only the application of flotation techniques for recovering barite from highly variable types of waste pond materials.
Georgia Waste Pond Sample
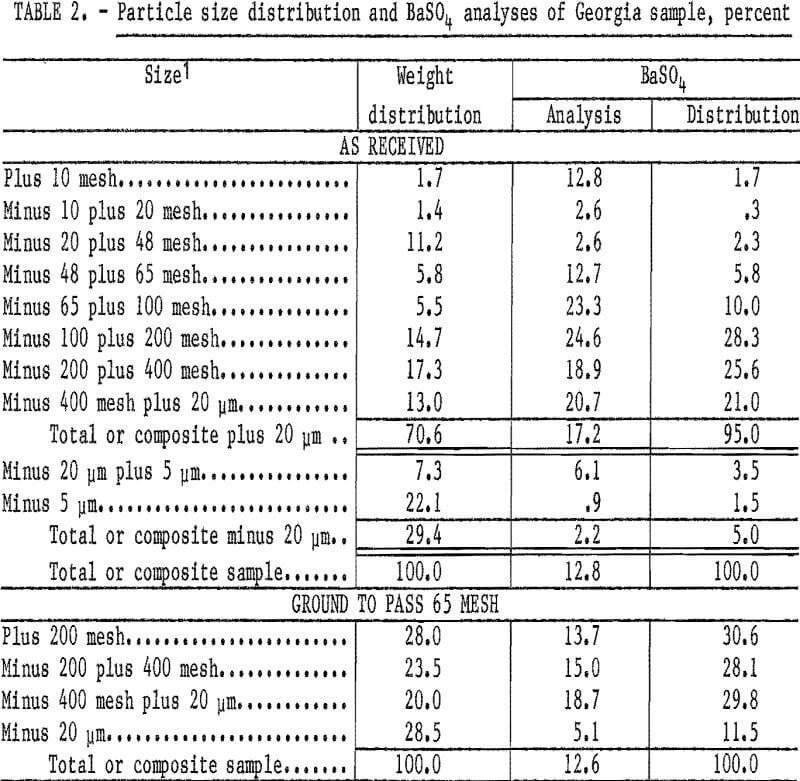
The waste pond sample from Cartersvllle, Ga., represented a distinct area in an old washer waste pond that contained 12.7 percent BaSO4, Microscopic examination indicated that the barite would be liberated at minus 65 mesh. Shown in table 2 are the particle size distribution and BaSO4 analyses of the sample, as received, and after the feed was ground to minus 65-mesh pulp for bench-scale flotation tests.
Preliminary flotation tests were made to determine the effects of varying the quantity of the reagent used for dispersion and pH control while maintaining a constant supply of the collector. The reagent used was sodium silicate, and the collector was sodium cetyl-stearyl sulfate, which was shown to be effective in previous studies. Both products are available commercially. The sodium silicate additions ranged from 0.5 to 6.0 pounds per ton of feed; the sodium cetyl-stearyl sulfate was maintained at 1.0 pound per ton of feed. After each sodium silicate addition, the ground pulp was conditioned for 2 minutes and then floated for 2 minutes in a rougher cell. Results of these tests (table 3) indicate the effectiveness of the increased sodium silicate on barite recovery. The BaSO4 analysis of the rougher-cell concentrates and the distribution of the BaSO4 in all fractions were calculated from the head sample, which contained 12.7 percent BaSO4.
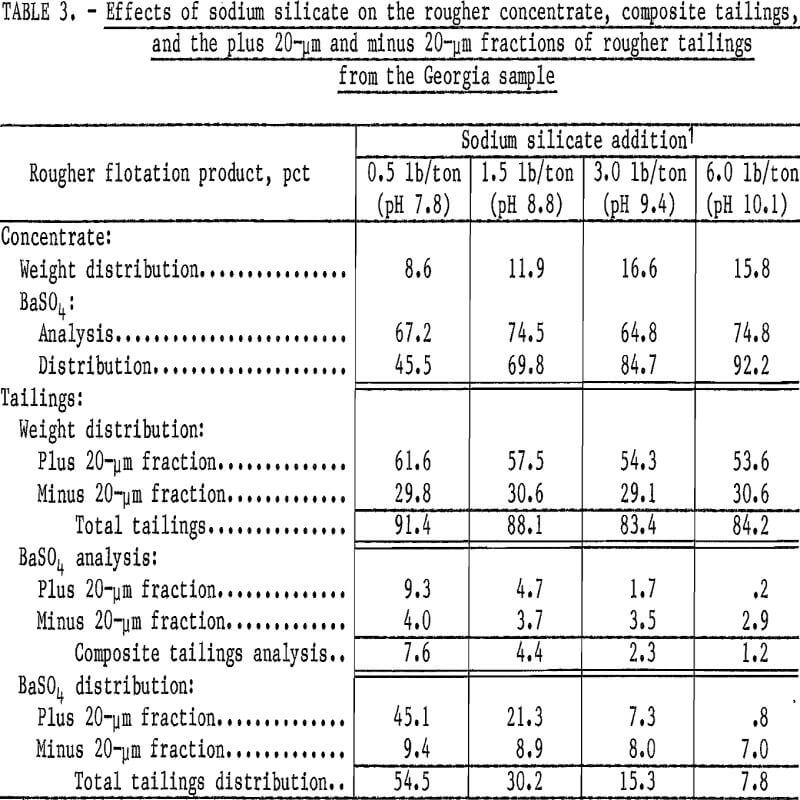
To determine the effect of the increased sodium silicate on the coarse and fine fractions, the rougher flotation tailings were also separated at 20 micrometers by sedimentation, and only these fractions were analyzed for BaSO4. The analysis of the tailings is a very accurate indicator of the particle sizes and the amount of barite reporting to the tailings. Increasing the sodium silicate rapidly improved the barite recovery from the plus 20-micrometer fraction, whereas only minimal improvement in recovery from the fine minus 20-micrometer fraction was obtained.
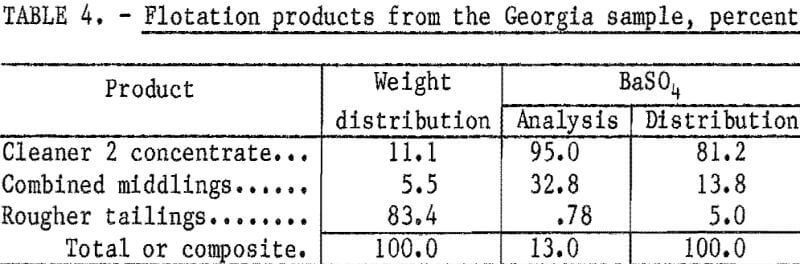
Based on these preliminary tests, additional tests were conducted to determine the number of cleaning stages required to produce a high-grade concentrate. Results of these tests (table 4), show that two stages of cleaning were sufficient to produce a concentrate meeting oil well drilling-mud specifications. The concentrate had a BaSO4 content of 95 percent and a specific gravity of 4.32; 81.2 percent of the barite in the feed was recovered.
Nevada Waste Samples
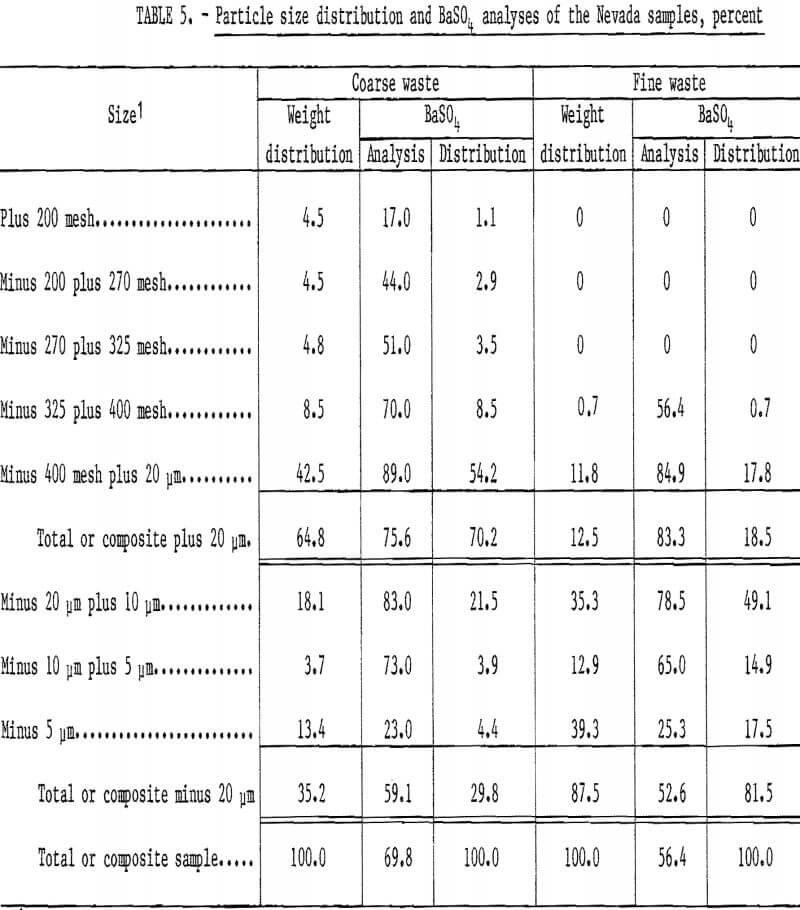
The two samples of barite waste material from a Battle Mountain, Nev., milling operation were sized by screening and sedimentation. Table 5 shows that one sample containing 69.8 percent BaSO4 was coarser than the other sample containing 56.4 percent BaSO4. Both of these samples were fine enough as received to be used as flotation feed without further grinding.
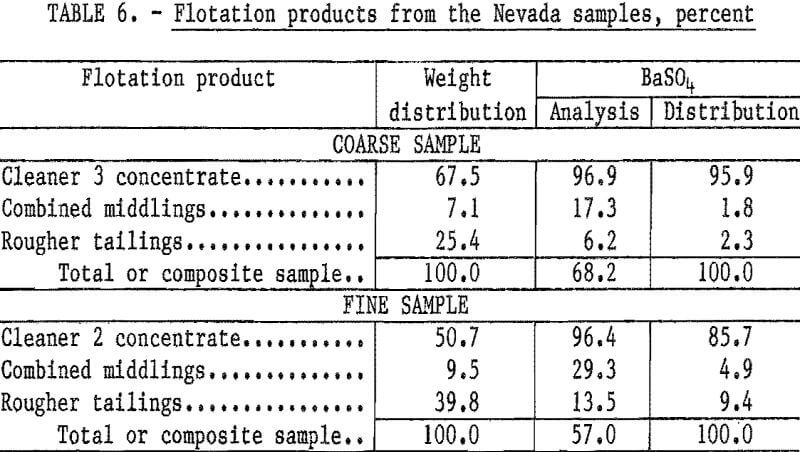
Treatment of the Nevada waste samples was relatively simple: dilute the samples to about a 30-percent solids content, condition 2 minutes with sodium silicate, condition 2 minutes with sodium cetyl-stearyl sulfate, float a rougher concentrate, and clean the rougher concentrate two or three times without additional reagents. Reagents required for treatment of the coarse sample were 0.65 pound of sodium silicate and 1.35 pounds of sodium cetyl-stearyl sulfate per ton. The cleaner 3 concentrate had a BaSO4 content of 96.9 percent and a specific gravity of 4.50; 96 percent of the barite in the feed was recovered. Complete results are shown in table 6.
Flotation of the fine sample required 1.0 pound of sodium silicate and 4.4 pounds of sodium cetyl-stearyl sulfate per ton. About 86 percent of the barite in the feed was recovered in the cleaner 2 concentrate, which had a specific gravity of 4.21 and a BaSO4 content of 96.4 percent.
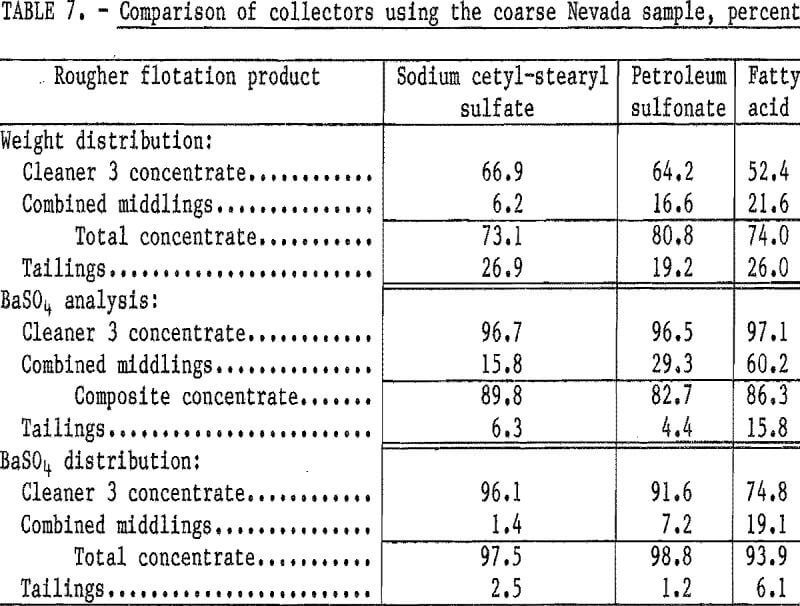
Flotation tests were conducted to compare the effectiveness of three different collectors on the barite in the coarse Nevada sample. The collectors used were (1) sodium cetyl-stearyl sulfate, (2) petroleum sulfonate, and (3) fatty acid; all commercially available. The tests were conducted using 1.0 pound of sodium silicate and 1.5 pounds of each collector per ton and a conditioning period of 2 minutes. The test results (table 7) indicate that, of the three collectors tested, sodium cetyl-stearyl sulfate achieved the highest barite recovery at substantially the same BaSO4 content.
Waste Pond Sample
The sample of Missouri waste pond material was received as a thick mud and contained 3.1 percent BaSO4. The sample was diluted to permit mixing and sampling; however, dilution to approximately a 30-percent solids content failed to yield a viscosity low enough to permit mixing. Therefore, sodium silicate was added incrementally to the slurry until the viscosity became low enough so that representative samples could be obtained. Approximately 20 pounds of sodium silicate per ton of solids was required to obtain sufficient dispersion and/or viscosity reduction to permit sampling.
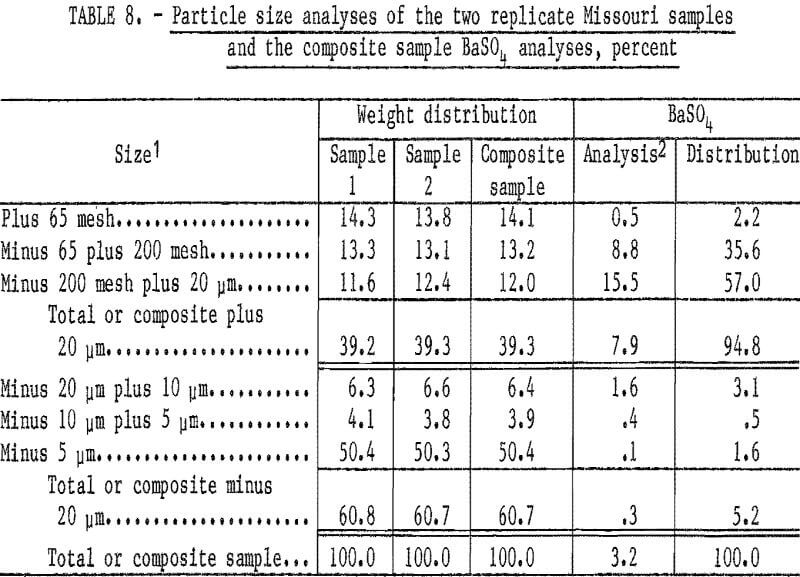
Two replicate samples taken from the semi-dispersed slurry were completely dispersed using additional sodium silicate and sized by screening and sedimentation. Since the sizing data (table 8) of the two replicate samples indicate good sampling techniques, the products from the two tests were combined for chemical analyses. Particle size and BaSO4 analyses of the combined sample show that 92.6 percent of the barite reported to the size fraction between 65 mesh and 20 micrometers.
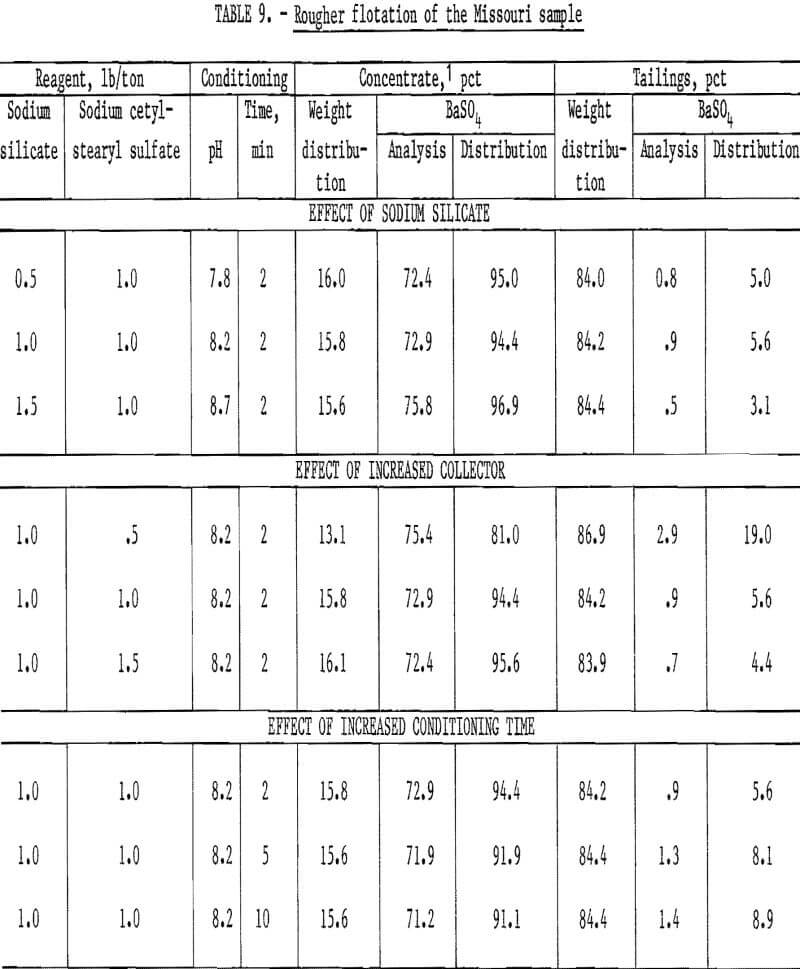
To study the effects of certain flotation variables on the minus 65-mesh plus 20-micrometer fraction, several pounds of this size fraction were isolated by sizing and sedimentation techniques. This fraction, which contained 12.2 percent BaSO4, was used as flotation feed to determine the effects of sodium silicate additions, collector concentration, and conditioning time. The tests were conducted using thoroughly deslimed flotation feeds with slightly alkaline pH, and reagent consumption ranged from 0.5 to 1.5 pounds per ton. The BaSO4 recovery in the concentrates obtained ranged from 81.0 to 96.9 percent. Only the rougher tailings were analyzed for BaSO4 the BaSO4 distribution values for the concentrates were calculated from the head analysis. The test results (table 9) show that there was only a slight increase in the amount of barite recovered with increased sodium silicate consumption and that the amount of barite recovered was inversely related to the conditioning time. The amount recovered decreased when less than 1.0 pound of sodium cetyl-stearyl sulfate per ton was used. The sodium silicate listed in table 9 reflects only that used in flotation and does not include the sodium silicate required for dilution purposes.
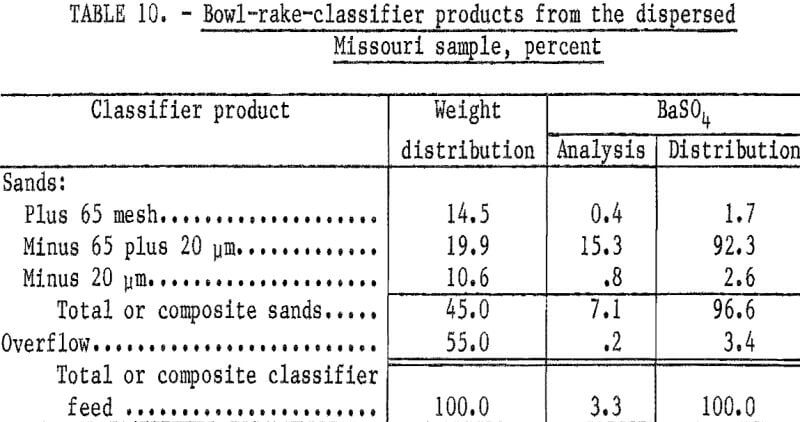
Particle sizing of the remaining semidispersed slurry was conducted using a small bowl-rake classifier. Sufficient tap water (average pH 9.3) was added to the classifier to yield a bowl overflow product containing only trace amounts of plus 400-mesh material; the pH of the tap water was sufficient to complete the dispersion of the slurry. Table 10 shows that excellent recovery of the barite was obtained in the classifier sands. A, sample of the classifier sands at 65 mesh to remove the barren oversize and partially deslimed at 20 micrometers was used for batch flotation tests. The classified feed was conditioned with 2.0 pounds of sodium silicate and 2.18 pounds of sodium cetyl-stearyl sulfate per ton at a pulp pH of 8.6. A rougher concentrate was floated and cleaned three times to yield the results shown in table 11. The third cleaner concentrate had a BaSO4 content of 96.6 percent and a specific gravity of 4.47; 86.1 percent of the barite in the flotation feed was recovered. Of the barite in the ore, 83.2 percent was recovered.

Illinois Waste Pond Sample
A sample of waste material was received from Cave-in-Rock, III. The sample was taken from the waste pond of a fluorspar beneficiation operation and contained 13.1 percent BaSO4 and 4.9 percent CaF2 (calcium fluoride). No efforts were made to recover the residual CaF2.
The sample was essentially minus 48 mesh, and microscopic examination indicated that most of the barite was liberated. Sedimentation sizing of the minus 20-micrometer fraction (table 12) indicated that the barite was uniformly distributed in the three size fractions and that no barren slimes could be rejected.

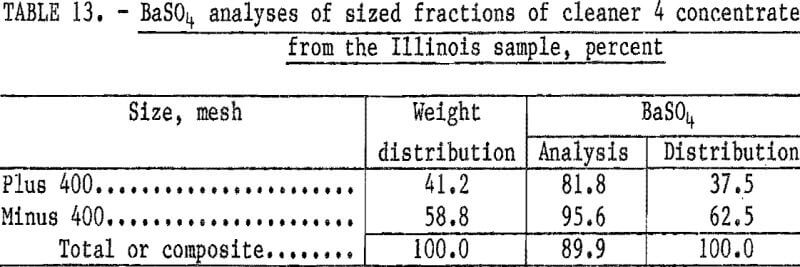
Flotation tests were conducted using 2.0 pounds of sodium cetyl-stearyl sulfate and 1.0 pound sodium silicate per ton. The barite was successfully floated; however, the concentrate contained significantly less BaSO4 than the 92 to 93 percent required for oil well drilling mud. The BaSO4 analyses of the concentrate and its sized fractions are shown in table 13.
The lower BaSO4 content of the plus 400-mesh fraction of the concentrate (table 13) was, at first, thought to result from the barite and gangue minerals not being liberated. Therefore, a number of flotation tests were conducted in which the sample was ground to pass 200 mesh prior to flotation. However, grinding to minus 200 mesh produced no significant improvement in the BaSO4 content of the concentrate.
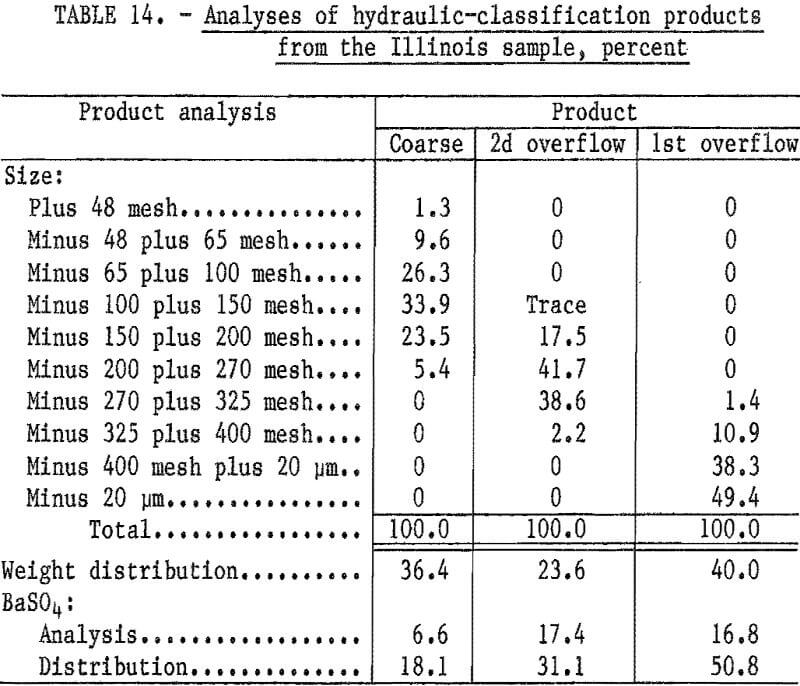
Hydraulic classification of the as- received sample was also conducted to determine if part of the sample could be rejected without additional grinding. The teeter-column hydraulic classifier was adjusted to produce an initial overflow product that was essentially minus 400 mesh. After removal of the minus 400-mesh fraction, the water flow was increased to yield a second overflow product that was essentially minus 100 mesh. The coarse product remained in the classifier.
Table 14 shows that rejecting the coarse product by hydraulic classification would discard 36 percent of the sample weight while losing only about 18 percent of the barite.
Flotation of each classifier product produced a high-grade concentrate with high recovery of the barite in the feed. From the coarse product, which was minus 48 plus 270 mesh, a cleaner 2 concentrate was produced that had a BaSO4 content of 97.3 percent, and 86.6 percent of the barite in that size fraction was recovered. From the second overflow product, which was minus 100 plus 400 mesh, a 2 concentrate was produced that had a BaSO4 content of 97.4 percent; 98.4 percent of the barite in that size fraction was recovered. Reagent consumption to produce the second overflow and coarse products was 0.5 pound each of sodium silicate and sodium cetyl-stearyl sulfate per ton. The first overflow product, which was substantially minus 400 mesh, required 2.0 pounds of sodium silicate and 1.0 pound of sodium cetyl-stearyl sulfate per ton. The cleaner 2 concentrate from the first overflow product had a BaSO4 content of 94.7 percent, and 94.1 percent of the barite was recovered. The composite cleaner 2 concentrate (table 15) had a BaSO4 content of 95.9 percent; 94.2 percent of the barite in the feed was recovered.
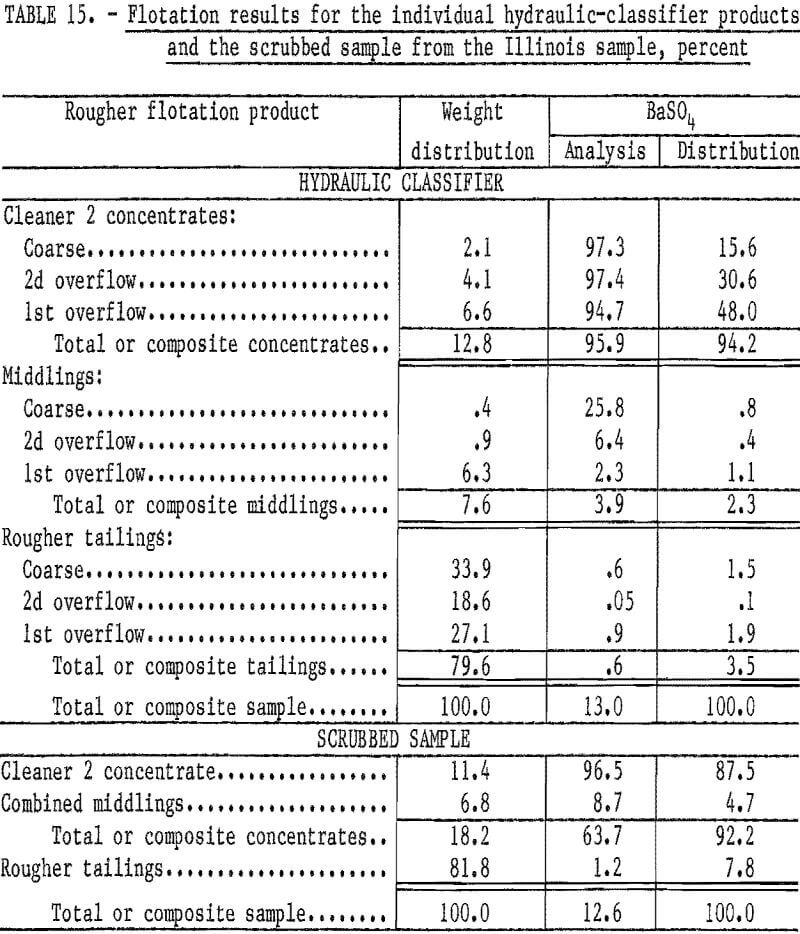
The results obtained by flotation of the three classifier products indicated that the initial idea of poor liberation of the barite would have to be discarded. It was hypothesized that the low BaSO4 content of the initial plus 400-mesh fraction (table 13) was caused by the presence of some residual reagent, flocculant, and/or other slightly soluble substances on the particle surfaces of the as-received sample. After removal of these substances during classification, the barite could be floated effectively.
To test this hypothesis, a sample of barite waste was attrition scrubbed for 5 minutes at a high percent solids. The scrubbed sample was diluted with tap water, allowed to settle, and the supernant water decanted. Flotation of the pulp with 0.5 pound of sodium silicate and 1.0 pound of sodium cetyl-stearyl sulfate per ton produced a cleaner 2 concentrate with a BaSO4 content of 96.5 percent while recovering 87.5 per-cent of the barite in the head sample. Results from this test (table 15) indicate that a high-grade concentrate, meeting oil well drilling-mud specifications, can be produced with high barite recoveries.
Discussion and Conclusions
Flotation tests were conducted using several types of barite waste materials with widely varying character, size, and barite content. These tests have shown that high-grade concentrates that meet oil well drilling-mud specifications can be produced from these widely divergent materials with high recovery of the barite. The waste materials had head analyses ranging from 3.1 to 68.7 percent BaSO4. The Georgia and Missouri samples had material larger than 10 mesh, while the fine Nevada sample was all minus 325 mesh. The plus 65-mesh and minus 20- micrometer fractions had to be removed from the Missouri sample to effect flotation, and the Illinois sample had to be scrubbed before effective separation could be accomplished. Flotation using sodium silicate and sodium cetyl-stearyl sulfate produced cleaned concentrates with BaSO4 contents ranging from 95.0 to 96.9 percent. The amount of barite reporting to the cleaned concentrates ranged from 81.2 to 95.9 percent of the barite in the feed. The specific gravities of the cleaned concentrates were all above 4.2, which is required in the specifications for well-drilling muds. Commercial use of this process can significantly increase the national barite reserves by yielding a more effective utilization of an important mineral commodity.
