The manufacture and use of nickel-cadmium alkaline batteries began to grow in the late 1950’s. During the period from 1966 to 1971, approximately 3 pct of the U.S. primary cadmium demand was consumed by battery manufacturers. From 1971 to 1975, the demand grew from 3 pct to 13 pct. It was estimated by the battery manufacturers (1) that by 1981 2.2 million pounds, or greater than 20 pct of the yearly U.S. demand for cadmium, will be consumed by battery manufacturers. The United States is dependent on Canada, Mexico, and Australia for greater than 60 pct of its primary cadmium supply.
At present, there is no commercial process being used in the United States to recover the total metal values from Ni-Cd battery scrap. Virtually all of the scrap recovered is shipped overseas, where it is processed and returned to this country as refined metals. Several scrap dealers are breaking the battery cells and hand-separating the positive and negative plates. The positive plates, containing 1 to 2 pct cadmium, are smelted in the United States to a high-ferro nickel alloy. The cadmium-rich negative plates are shipped overseas.
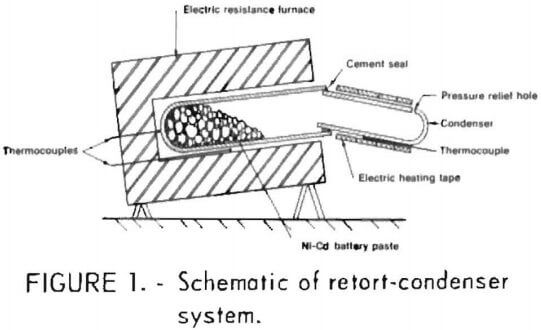
As part of its efforts in secondary metals recovery, the Bureau of Mines, U.S. Department of the Interior, pursued this research, which has led to the development of a pyrometallurgical method for recovering metallic cadmium and a nickel-iron residue low in cadmium (fig. 1). This paper is a progress report of the laboratory tests that have been conducted to date. Work is continuing on scaling up the method to test 10- to 20-pound charges of battery scrap. At the time of this investigation, there were only three other known methods: A hydrometallurgical method developed in 1971 by the Bureau of Mines, a sulfuric acid leach and electrolytic recovery method, and a pyrometallurgical method based on a French patent that fave no details.
This research has progressed to larger scale testing of 10- to 20- pound charges of Ni-Cd battery scrap. The small laboratory tests have successfully demonstrated that the cadmium in the residue can be consistently reduced to 0.05 pct or less by operating at 900° C and atmospheric pressure for 2 hours using 2.5 pct carbon. Although the initial large-scale test did not reduce the cadmium to less than 0.1 pct, no difficulties are anticipated in achieving this level and producing a cadmium condensate with low impurities. Work will be continued on the large-scale retort.
