Table of Contents
At the present time, zinc is produced commercially by a roast-leach zinc electrowinning process. The primary source is high-grade concentrates of the mineral ZnS. Concentrates must contain low levels of iron to avoid formation of zinc ferrites during roasting because zinc ferrites are not amenable to leaching and cause decreased zinc recovery. Also, SO2 is produced during roasting and must be scrubbed from flue gases to prevent air pollution. Progressively more stringent standards for SO2 emission and interest in processing more complex sulfide deposits have prompted research to develop hydrometallurgical processes as alternatives to the conventional roast-leach treatment of ZnS concentrates. Most complex sulfides are lower grade, contain more impurities, and are not amenable to treatment by existing techniques.
One method that shows promise is chlorine-oxygen leaching. This method is particularly well suited to treating ores that are high in iron content and yields a concentrated ZnCl2 solution. Metallic zinc may be recovered from the solution by electrowinning; however, it is of unsatisfactory quality. There are no known techniques for producing a satisfactory zinc plate from chloride solutions. As an alternative, a molten-zinc product can be produced by molten-salt electrolysis of anhydrous ZnCl2.
Molten-salt electrolysis of ZnCl2 yields a molten-zinc product, which can be tapped from the cell in a continuous manner. Since the molten-salt cell operates at a temperature of 500° C, the ZnCl2 feed must be anhydrous to avoid explosive release of steam. Also, the presence of O2 will cause corrosion of the carbon electrodes.
Recovering anhydrous ZnCl2 from aqueous leaching solutions has proven in the past to be an expensive, energy intensive, and difficult task. During evaporative crystallization, the high solubility of ZnCl2 results in viscous mother liquors that are very difficult to filter. Additionally, while ZnCl2 is easily dried, the anhydrous salt is deliquescent and maintenance of anhydrous conditions is difficult. Shanks noted that some types of ZnCl2, purchased as feed for molten-salt electrowinning studies, were less hygroscopic than others. Analysis indicated the presence of NH3, suggesting the presence of a double salt or an ammine chloride. Development of an efficient method for producing a zinc-ammonia salt would yield a less deliquescent feed material for molten-salt electrolysis.
Previous studies by Kruesi have shown that zinc-ammonia salts can be generated through solvent extraction techniques. ZnCl2 is extracted from an aqueous solution into an organic phase and is then stripped into a solution from which an ammine chloride can be crystallized. Kruesi proposed two methods for stripping zinc from the organic phase. One method employed ethylene glycol and the other employed an aqueous ammoniacal chloride solution as the stripping solution. The ethylene glycol solution was contacted with gaseous ammonia to crystallize zinc ammine chloride followed by heating of the recovered precipitate to remove ammonia. To avoid contamination of the ZnCl2 product by residual ethylene glycol, Kruesi recommended recrystallization of the zinc ammine chloride prior to thermal treatment. In the second method, the organic was stripped at elevated temperature. Zinc ammine chloride was crystallized from the stripping solution by cooling. The crystalline product was recovered by filtration; the mother liquor composition was adjusted by addition of ammonium hydroxide and was recycled to the stripping circuit.
If an ammoniacal ZnCl2 salt could be precipitated directly from an aqueous leach solution, the solvent extraction step could be avoided. This would simplify the recovery sequence by avoiding either the need for recrystallization or concern over premature crystallization during solvent extraction. As part of its program to assure an adequate supply of metals and minerals from domestic sources, reduce environmental pollution, and conserve energy, this study was done to demonstrate technical feasibility for crystallization of an ammoniacal ZnCl2 salt directly from an aqueous leach solution and conversion to anhydrous ZnCl2. If solvent extraction can be avoided, there should be a cost savings in the process.
Methods and Procedures
Solutions used in crystallization tests were prepared from reagent-grade salts by dissolving weighed quantities in deionized water. All chemicals added to promote crystallization were reagent grade. Spray drying tests were done in a minispray dryer. Precipitated products were recovered by vacuum filtration and dried at 110° C.
Ammonia sparging tests were performed in a 3-L water-jacketed borosilicate glass resin kettle having four standard taper joints in the lid (fig. 1). Temperature was controlled by a refrigerated circulating bath (minus 20° to plus 300° C) connected by rubber tubing to the sparging kettle water jacket. Temperature was monitored with a Chromel-Alumel thermocouple and a digital temperature indicator.
Anhydrous NH3 was delivered through a pressure regulator and flowmeter to a fritted glass tube located just above the propeller in the sparging vessel. The ZnCl2 solution was stirred with a three-bladed, steel-reinforced polyethylene stirrer (18-in length, ¼-in shaft diameter, 1-¾-in propeller diameter, and 45° pitch) connected by a flexible shaft assembly to a variable speed 1/40-hp electric motor capable of 0- to 6,000-rpm armature shaft speed. A standard taper inlet adapter with a slightly oversized ¼-in hole was used as the centering device and bushing for the stirrer. The bushing was not made gas tight to avoid pressure buildup in the system. The lime treatment was performed in 2-L beakers using magnetic stirring. The pH was monitored with a pH meter.
The chloride and NH3 concentration in solutions and crystallized salts were obtained by wet chemical methods. Metal analyses were obtained by atomic absorption spectroscopy or inductively coupled plasma spectroscopy. Crystal structures of precipitates were determined by X-ray diffraction. O2 content of precipitates was determined by inert gas fusion at the Bureau’s Albany Research Center. Differential thermal analysis was done on ammoniacal salts to determine the temperatures at which ammonia could be removed.
Calcination tests were done in a 3-L resin kettle fitted with a heating mantle, and the vapors were vented through a scrubber (fig. 2). Temperature was controlled using a Chromel-Alumel thermocouple, located over a porcelain

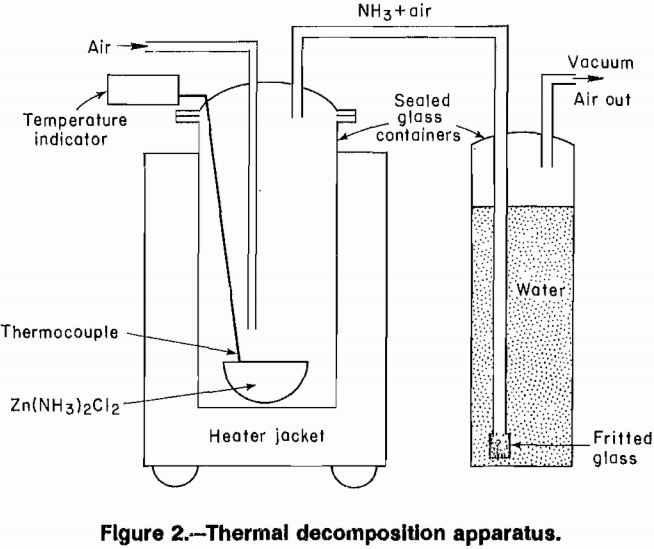
evaporating dish containing the sample. Two glass tubes were introduced through sealed, tapered joints in the lid of the resin kettle. One tube extended to just above the porcelain dish, providing outside air to sweep away reaction products. The second tube, near the top of the vessel, carried the reaction products to the scrubber. The scrubber consisted of a gas dispersion tube immersed in water, and the reactor was evacuated to draw reaction products from the calciner. Water from the scrubber was titrated for NH3 content at the end of each test.
Results and Discussion
Screening Tests
Preliminary screening tests were done to determine the conditions under which ammoniacal ZnCl2 salts could be formed. Crystalline products from the tests were used to obtain X-ray diffraction characterization and differential thermal characteristics.
The first technique investigated was one previously used to produce zinc ammine chloride or magnesium ammine chloride. An aqueous solution of ZnCl2 was dissolved in ethylene glycol and sparged with NH3 at 20° C. Solution progressed from colorless to milky white as sparging progressed and crystallization commenced. Separation of crystals from the ethylene glycol solution was difficult because solution viscosity and filtered crystals contained entrained organic material. Analysis of crystalline products showed that Zn(NH3)2Cl2 was the major product with some amorphous material and a trace of ZnCl2-2NH4Cl.
Formation by evaporative crystallization was another method evaluated. To test the feasibility of this approach, 1 mol/L ZnCl2 solutions were oven evaporated under different conditions (table 1). When ZnCl2 solutions were evaporated, a contaminant appeared in the product. The contaminant had a distinct but unknown X-ray diffraction pattern. Adding 1 mol/L NH4Cl to the test solutions produced an NH4Cl double salt plus hydroxychloride under atmospheric conditions and zinc oxide (ZnO) with a trace of the double salt when dried under vacuum. In tests using a spray dryer, no crystals were produced by aspirating ZnCl2 solution with air in an attempt to produce ZnCl2 crystals (table 2). Aspirating a solution of ZnCl2 and NH4Cl with air produced a mixture of double salts and amorphous material. Aspirating either solution with ammonia produced zinc ammine chloride. Some additional tests were done using ZnCl2 recovered from solution by vacuum drying. The recovered salts were exposed to an NH3 atmosphere for 1 h at 20° C. Part of the ZnCl2 was converted to (NH4)2ZnCl4. The converted material was ground to minus 200 mesh and again exposed to an NH3 atmosphere for 1 h at 20° C. The final product was Zn(NH3)2Cl2.
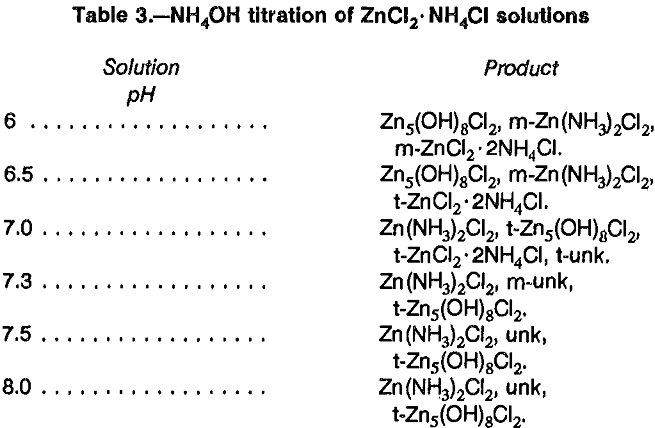
Preliminary tests were also performed to crystallize zinc ammine salts directly from solution by titrating 1 mol/L ZnCl2 solution with NH4OH. Tests done at ambient temperature produced ZnO with minor Zn(NH3)2Cl2 at pH 7.0. Repeating the test at 60° C yielded ZnO and two unidentified phases at pH 6.2. Titrating a mixture of ZnCl2 and NH4Cl at ambient temperature yielded different crystalline products as pH increased from 6 to 8 (table 3). Oxychlorides were the major products, up to pH 6.5, and were present at all pH levels tested.
Having found that at least three different zinc ammine salts may be crystallized from ZnCl2 solutions, it became important to determine whether any of the three would be more desirable as feed to the fused-salt electrolysis cell. To this end, differential thermal analysis was done on each salt (figs. 3-4). The broad endothermic peak shown for each salt indicates loss of ammonia, while a second heating cycle showed only endothermic peaks at temperatures
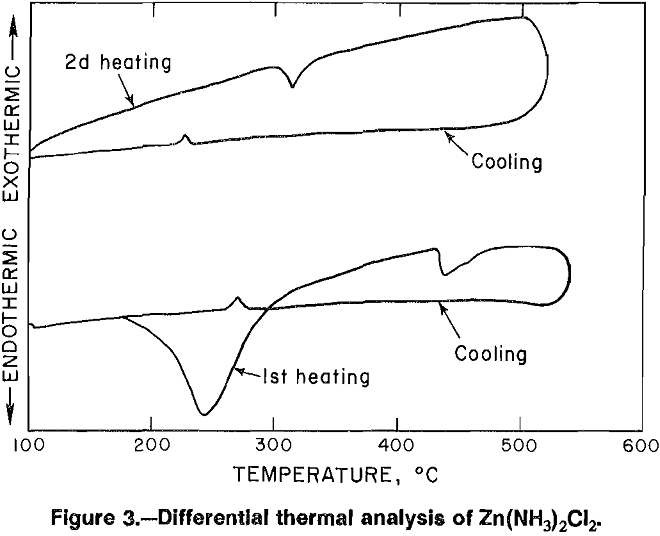
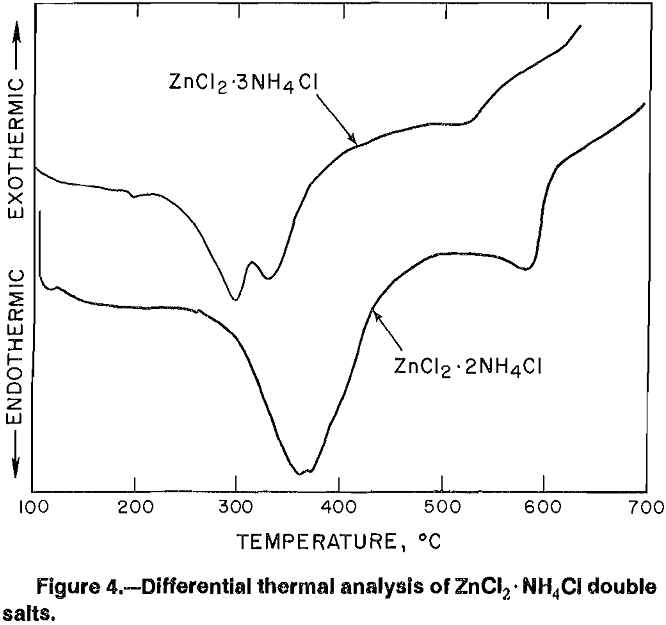
corresponding to melting and boiling of ZnCl2. Ammonia removed from ZnCl2-2NH4Cl and ZnCl2-3NH4Cl was in the form of NH4Cl, which deposited as a white film on any cool surface. It was felt that recovery of ammonia as NH4Cl using the two double salts would complicate a continuous process; therefore, further research was concentrated on crystallizing Zn(NH3)2Cl2, from which NH3 is recovered as a gas.
Sparging Tests
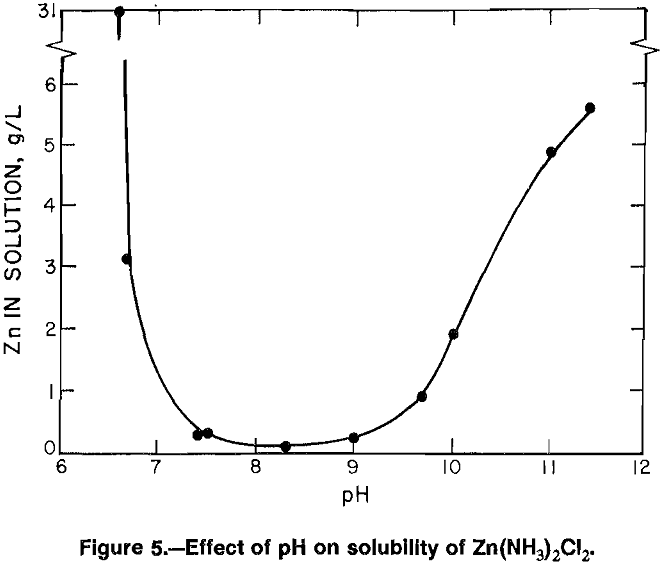
Since the ammine chloride was formed both by spray drying using NH3 carrier and by precipitation using NH4OH, it was likely that it could also be formed by sparging a ZnCl2 solution with gaseous NH3. Such an approach has the advantages of crystallizing a zinc product directly from solution, avoiding the costly water removal required by evaporative techniques, and facilitating the recovery of NH3 gas for recycle. To avoid excess use of NH3, it was necessary to determine the minimum time required for sparging to reach completion. A convenient way to determine this was to do a series of tests to measure the effect of pH on Zn(NH3)2Cl2 solubility, which established minimum solubility of the salt.
A Zn(NH3)2Cl2 crystalline product (38 pct Zn) from previous tests was used. When Zn(NH3)2Cl2 was added to deionized water, the pH rose from 4.0 to 7.5 and 0.25 g/L Zn dissolved in solution (fig. 5). Solution samples were taken at different pH values and analyzed for zinc content. When NH4OH was added to the solution to raise the pH to 8.3, the zinc in solution decreased to 81 µg/mL. With continued increase of pH, zinc in solution increased from 0.19 g/L at a pH of 9.0 to 5.6 g/L at a pH of 11.4 where all the crystals were dissolved.
To obtain information at lower pH values, HCl was added to a saturated solution of Zn(NH3)2Cl2. Zinc in solution increased from 0.285 g/L at a pH of 7.5 to 3.1 g/L at a pH of 6.7. Continued addition of HCl did not decrease solution pH, but continued to dissolve zinc until all of the Zn(NH3)2Cl2 had gone into solution. From these results, it appeared that sparging to a pH range of 7.5 to 9.0 would precipitate most of the zinc. A pH of 8.3 would be desirable.
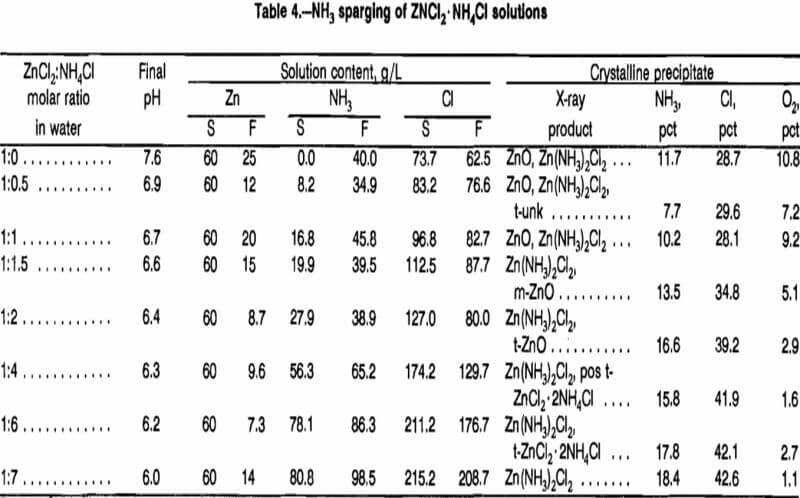
Initial NH3 sparging tests were done on 2-L samples of 1 mol/L ZnCl2 at 20° C. Samples of crystalline products were taken as the test progressed and the pH was recorded. Analysis of the products showed that most of the precipitate was ZnO, an undesirable feed for a molten-salt electrolysis cell. It was observed, however, that continued sparging dissolved all of the precipitate. By acidification of the solution containing dissolved ZnO to pH 7.2 with gaseous HCl, Zn(NH3)2Cl2 was precipitated as the major product. Because large quantities of NH3 and HCl are consumed using this method, tests were done adding NH4Cl to the ZnCl2 solution to obtain the same result.
A series of tests was done at 20° C, starting with 1 mol/L ZnCl2 containing NH4Cl at zinc-to-NH3 molar ratios of 1:0 to 1:7. Tests were considered complete when no additional crystallization from solution could be noted as more NH3 was sparged and pH increased. The compositions of the initial solution and final filtrates were determined as were the compositions of crystalline products (table 4). Evaluation of results showed that ZnO was a major product until the initial ZnCl2-to-NH4Cl molar ratio exceeded 1. In the test at a ratio of 1:0 ZnCl2:NH4Cl, zinc concentration in the final solution is higher than predicted from figure 5 data. The presence of ZnO in the precipitate indicates that some of the ZnCl2 was hydrolyzed and NH4Cl was generated. From the results of the tests, it is apparent that the presence of NH4Cl in the starting solution increases zinc solubility. Some ZnO was still detected when the ZnCl2-to-NH4Cl ratio was 1:2, but O2 analysis was 2.9 pct, a level considered acceptable for feed to a fused-salt electrolysis cell. Larger ratios of NH4Cl lowered 02 content to 1.1 pct. The value of 2.7 pct for the ratio of 1:6 is thought to be due to incomplete drying of the sample prior to analysis. Thus, O2 content is high because of the presence of H2O. For the ratio of 2 mol NH4Cl per 1 mol ZnCl2, which is the minimum needed to reduce 02 content to an acceptable level, 15 pct of the zinc will remain in sparging mother liquors containing 2.3 mol/L NH4Cl. The sparging mother liquor will need to either be treated for NH4Cl recovery or recycled to leaching to avoid unacceptable reagent consumption.
If the mother liquors are recycled to a Cl2-O2 leach, there is concern that NH4Cl would be lost by decomposition to N2 and H2. If inhibition of ZnO species was due to increased chloride concentrations, using a more stable chloride salt could provide the concentrations required to control 02 levels. To study the effectiveness of a more stable salt, tests were done in which NH4Cl was replaced with calcium chloride (CaCl2) and mixtures of CaCl2 and NH4Cl. Use of CaCl2 yielded crystalline products of ZnO salts. The CaCl2-NH4Cl mixtures yielded Zn(NH3)2Cl2, along with ZnCl2 · 2NH4Cl, ZnO, and amorphous phases. As the substitutions for NH4Cl were not effective, all further tests employed NH4Cl.
Effect of Impurities
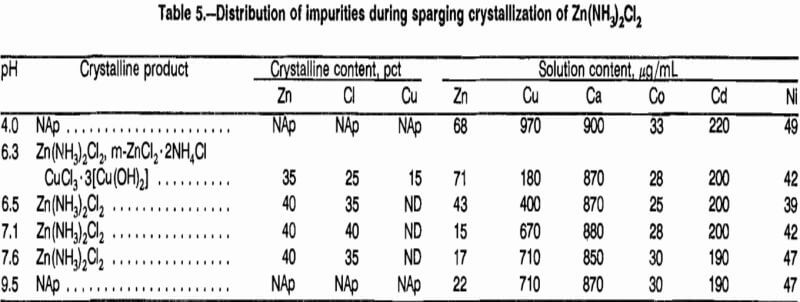
Having demonstrated a technique for crystallization of Zn(NH3)2Cl2 directly from aqueous solutions of ZnCl2, attention turned to the distribution of potential impurities in actual leaching solutions. A simulated leaching solution was prepared based on the composition stated in earlier work. The solution contained, in grams per liter: 97 Zn, 4.5 Fe, 2.0 Cu, 1.0 Ca, 0.15 Cd, 0.11 Ni, 0.022 Pb, and 0.041 Co. The pH was 4.2. Sparging up to pH 5.6 resulted in amorphous precipitates containing all of the copper and iron. Above pH 5.6, zinc ammine chlorides were obtained, which, when analyzed by X-ray fluorescence spectroscopy, contained no impurities. The results indicate that the major contaminating impurities could be removed by adjusting the solution pH to 4 to 5, thus precipitating iron and copper prior to crystallizing Zn(NH3)2Cl2.
Since hydrous ferric oxide is formed during sparging and is known to act as a collector for other metal ions, additional tests were done on simulated leaching solutions containing no iron to determine whether copper contamination would still occur. Precipitates were removed at intermediate pH values and analyzed (table 5). The results show that copper precipitates below pH 6.3 as a hydroxychloride, but redissolves at higher pH to yield uncontaminated Zn(NH3)2Cl2. Thus, the sparging technique may be used to recover and purify zinc from aqueous solutions. If iron is present, it must be removed in a separate step. Solution analyses indicate that impurities such as calcium, cadmium, cobalt, and nickel remain in sparged mother liquors and, therefore, would not contaminate crystallized products.
Lime Treatment of Mother Liquor
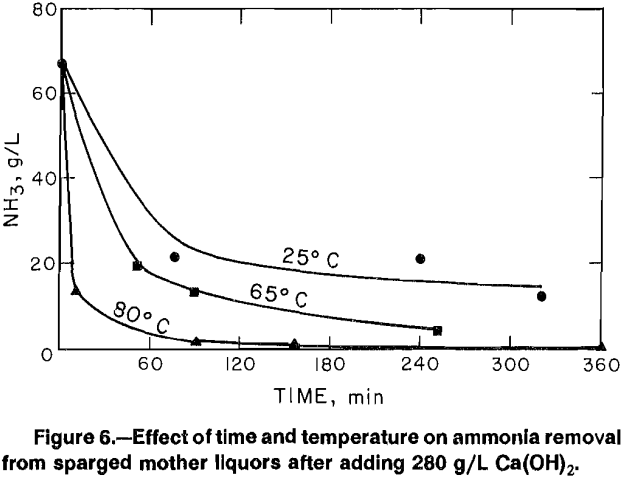
While results of sparging tests confirmed that Zn(NH3)2Cl2 could be crystallized from solution, the need to add NH4Cl yielded mother liquors containing up to 15 pct of the initial zinc and large amounts of NH4Cl. Recycling of the mother liquor to leaching would recover the zinc, but it was feared that the leaching conditions would decompose the NH4Cl. Additions of Ca(OH)2 to the mother liquors would convert NH4Cl to CaCl2, which would be compatible with Cl2-O2 leaching conditions and allow NH3 to be recovered as a gas. Tests were done in which Ca(OH)2 was added to a sparging mother liquor at 25°, 65°, and 80° C for a period up to 6 h to determine conditions for recovering NH3. Since Ca(OH)2 addition also precipitates zinc, its behavior was also monitored.
Figure 6 shows the decrease in ammonia content versus time for the three temperatures for a Ca(OH)2 addition of 280 g/L of mother liquor. The addition level of Ca(OH)2 was calculated as being sufficient for conversion of all of the NH4C1 to CaCl2 and all of the ZnCl2 to ZnO. Zinc behavior is shown in figure 7.
At 25° C, the NH3 decreased from 66.5 to 12.7 g/L and the zinc content decreased from 17 to 3.4 g/L in 320 min. At 65° C, the NH3 decreased to 4.1 g/L and the zinc content decreased to 190 µg/mL in 260 min. At 80° C, the NH3 decreased to 0.29 g/L and the zinc content decreased to 43 µg/mL in 160 min. Higher temperature not only decreased the amounts of both NH3 and zinc, but yielded faster reaction times. The rapid decrease in both NH3 and zinc concentrations at 80° C suggested that less Ca(OH)2 may be required.
Tests using 210 and 140 g Ca(OH)2 at 80° C yielded almost identical results to those shown in figures 6 and 7. Additions of 70 g Ca(OH)2 yielded lower removal of both zinc and NH3. A more detailed study of Ca(OH)2 additions was not done at this time because analysis of precipitated material showed that CaCO3 was present. Since fresh reagent-grade Ca(OH)2 had been used, any carbonate apparently resulted from absorption of CO2 from air by the ammoniacal solutions. Such absorption would be variable and uncontrolled so that the amount of added Ca(OH)2 available for reaction with NH4Cl could not be accurately measured. Age of solutions would, therefore, be a factor in Ca(OH)2 requirements.

Thermal Decomposition of Zn(NH3)2Cl2
For Zn(NH3)2Cl2 to be a practical intermediate for recovering anhydrous ZnCl2 from solution, it must be possible to remove and recover the NH3. To define the decomposition step and feasibility of producing NH3 for recycle, tests were done over a range of temperatures and times (table 6). Ammonia removal was based on weight loss versus the calculated weight of NH3 in the sample. Ammonia recovery was based on NH3 reporting to the trap versus weight loss.
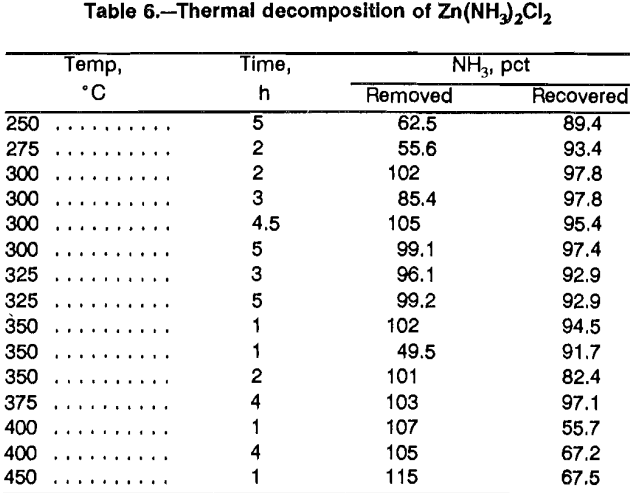
While there is some variability in the NH3 removal results, certain trends are apparent. At temperatures less than 300° C, the weight loss shows that NH3 was incompletely removed, but most of the liberated NH3 was recovered. At temperatures of 300° to 375° C, most of the tests resulted in near complete NH3 liberation and recovery. At temperatures of 400° to 450° C, NH3 was liberated, but recovery was incomplete. At the elevated temperatures, low NH3 recovery was attributed to decomposition of NH3 to N2 and H2. Confirmatory evidence for NH3 decomposition was obtained by testing gas mixtures with a sensor to detect explosive mixtures. Explosive mixtures were detected for tests at 400° to 500° C, but not at lower temperatures. While NH3 may form an explosive mixture, 16 pct NH3 is required as opposed to 4.1 pct for H2.
The major inconsistency in the data was the replicate test at 350° C for 1 h. The test in which 49.5 pct of the NH3 was liberated was done on a charge that contained 58.6 pct more material and was the largest sample tested. The results were included to demonstrate that heat transfer in the salt is an important parameter for NH3 liberation.
Process Flowsheet
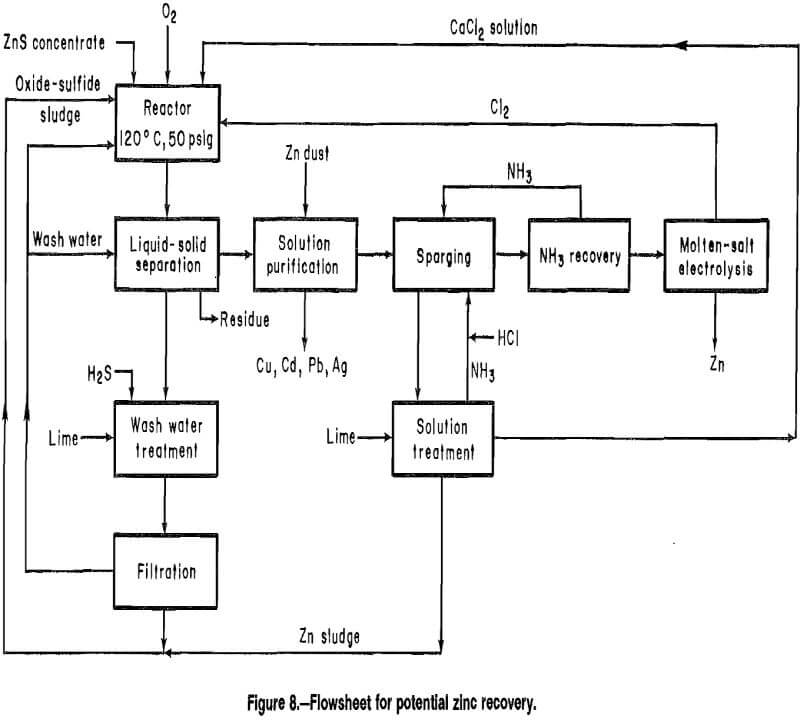
To illustrate how the ZnCl2 recovery steps discussed in this report could be used as part of a zinc recovery process, a conceptual flowsheet developed from earlier work was modified to include the NH3 sparging treatment steps (fig. 8). Although the modification replaced the ZnCl2 crystallization step with three separate operations, the new flowsheet offers several advantages. Foremost among the advantages is that the difficult and costly evaporative crystallization step is eliminated. Additionally, while the zinc cementation from the original flowsheet was retained, the investigation of the effect of impurities on Zn(NH3)2Cl2 recovery demonstrated that it may be possible to eliminate this step unless recovery of those metals precipitated by zinc is desirable or unless such impurities build up to undesirable levels during recycle.
Inspection of the flowsheet shows a reasonably closed processing sequence in which the consumed reagents are lime, O2, H2S, and HCl. A potential buildup of CaCl2 must be considered and could be handled by a bleed-stream from the recycled CaCl2 solution. Most of the added calcium would report to the residue as CaSO4 produced in the reactor. Buildup of impurities, such as copper, cadmium, and nickel could also be controlled by a bleedstream. The metals could be recovered by zinc cementation of the bleedstream in a manner similar to the method described in earlier work. All aqueous streams are treated and recycled so that the only water losses from the process would be wash water in the leached residue.
Conclusions
This investigation has demonstrated that Zn(NH3)2Cl2 can be made from ZnCl2 solutions or ZnS leaching solutions containing other metallic ingredients, by adding NH4Cl to the solution and sparging with NH3 to a pH of 6 to 7.5 at 20° C. After the zinc ammine chloride is precipitated from solution, the spent liquor can be reacted with Ca(OH)2 to remove remaining zinc and NH3 from solution. The Zn(NH3)2Cl2 can be heated from 300° to 375° C for 1 to 4 h to drive off the NH3 for recycling, and the ZnCl2 can be used as feed to a fused-salt electrolysis cell.
The ZnCl2 recovery technique resolves some of the difficulties encountered in recovering zinc from ZnS concentrates by chlorine-oxygen leaching. The need to concentrate leaching solutions by evaporation is eliminated and an easily filterable, crystalline precipitate is produced. The Zn(NH3)2Cl2 precipitate is less deliquescent than ZnCl2, which would facilitate storage of salts intended to be used as feed to a fused-salt electrolysis cell, thus increasing operating flexibility in the leaching system.
