Table of Contents
The preparation of ductile vanadium from vanadium carbide was investigated by the Bureau of Mines as a part of a research program on the extraction and utilization of vanadium resources. The vanadium was extracted from the vanadium carbide by electrolysis in a molten-salt electrolyte protected from oxidation by a helium atmosphere.
The preparation of relatively pure ductile vanadium was not accomplished until 1927. According to Rostoker, ductile vanadium with hardness below 90 Rockwell B should contain less than 0.2 percent combined oxygen and nitrogen. He also stated that a carbon content of vanadium below 0.25 percent had little effect on the ductility. Loria and coworkers and Ramsdell and coworkers have reported on the effects of carbon, nitrogen, and oxygen in electrorefined vanadium.
Ductile vanadium has been prepared by several methods. Higher purity ductile metal (99.9 percent) has been prepared by the alumino-thermic reduction of vanadium pentoxide (V2O5) followed by an electron-beam refining process, and by the metallothermic reduction of vanadium tri-chloride (VCl3). Lei, Cattoir, and Sullivan reported on a molten-salt electrolytic process for producing ductile vanadium from 90-percent-grade vanadium.
A possible low-cost starting material for the production of vanadium is vanadium carbide. This material is being produced commercially by Union Carbide Corp. as Carvan, primarily for use as a replacement for ferrovanadium in the steel industry. The preparation of a vanadium carbon alloy, for use as a steel addition, from vanadium oxides was described by Downing and Merkert. The material used in this investigation was a V2C-type vanadium carbide which contained approximately 85 percent vanadium and 10 percent carbon, with the balance chiefly oxygen, iron, and chromium. The preparation and the physical, chemical, and thermodynamic properties of vanadium carbide have been given by Storms.
The carbothermic reduction of vanadium oxides to prepare vanadium requires both high temperature and high vacuum, owing to the formation of various stable vanadium oxide and carbide phases. Kroll and Schlechten reported that they obtained 94.5 percent vanadium with high interstitial content by the carbothermic process with a reduction temperature of 1,853° K and a minimum reduction pressure of 10 -6 atmosphere. A. Yu. Polyakov described the conditions by which ductile vanadium was prepared by carbothermic reduction of vanadium trioxide (V2O3).
This report describes an electrolytic process by which ductile vanadium was prepared from V2C carbide.
Theoretical Considerations
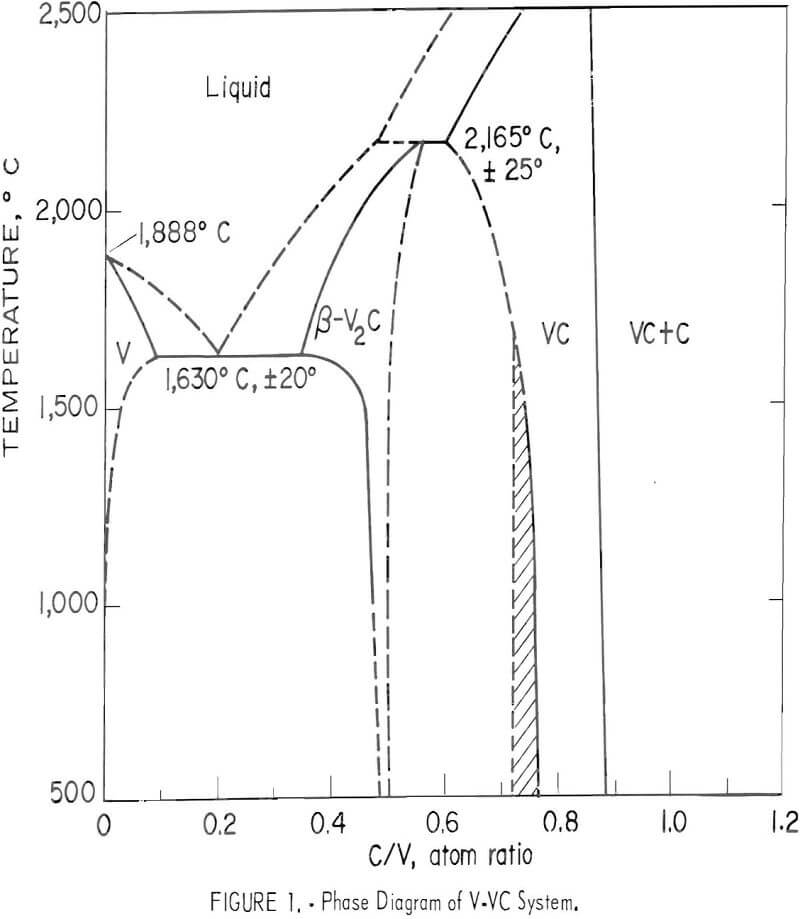
The phase diagram of the vanadium-vanadium carbide system by Storms in the temperature range between 500° and 2,500° C is given in figure 1. The reaction for extracting vanadium from vanadium carbide,
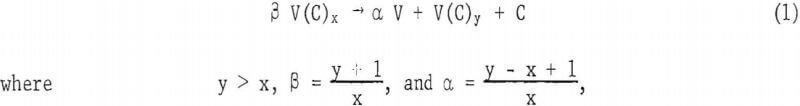
is governed by the partial molal free energy of vanadium which varies with the composition of different vanadium carbide phases. Partial molal free energy data for vanadium are not available for the vanadium-vanadium carbide system.
The energy required for the extraction may be estimated from the equilibrium thermodynamics of various stable carbide phases of the vanadium-vanadium carbide system. Mah showed that Δ F° 1,000° K for V2C is -34.2 kcal/mole and for VC0.88 is -23.0 kcal/mole. Accordingly, the electromotive force necessary for decomposition of V2C at 1,000° K is 0.74 abs volt, and
0.5 abs volt for VC0.88. From the phase diagram, two possible mechanisms for the extraction of vanadium are postulated. The first is the direct decomposition of V2C to vanadium and carbon as represented by
V2C → 2 V + C; Δ F° = +34.2 kcal………………………………….(2)
The second mechanism involves the formation of VC0.88 and free carbon as products when vanadium is removed by electrolysis from the V2C which leads to an increase in the carbon content of the vanadium-vanadium carbide system. That is,
V2C → V + VC0.88 + 0.12 C; Δ F° = +11.2 kcal…………………….(3)
By comparing the difference in the free energy values, the second mechanism appears more favorable.
The phase diagram indicates that the carbon-saturated vanadium carbide phase is VC0.88. Further extraction of vanadium from this phase should be possible since it will not increase the carbon content of the VC0.88 phase. The reaction and free energy change are
VC0.88 → V + 0.88 C and Δ F° = + 23 kcal, respectively………………………..(4)
The energy requirements for reactions 3 and 4 to proceed are furnished by electrolysis. The proposed electrolytic reaction for the preparation of vanadium by electrolysis of vanadium carbide in a molten electrolyte containing vanadium dichloride (VCl2) is as follows:
Anode
VCl2 + Cl- → VCl3 + e-…………………………………………………………..(5)
and 2 VCl3 + V2C → 3 VCl2 + VC0.88 + 0.12 C……………………….(6)
or V2C + 2 Cl- → VCl2 + VC0.88 + 0.12 C + 2 e-……………………..(7)
Cathode
VCl2 + 2 e- → V + 2 Cl-……………………………………………………….(8)
The net cell reaction is similar to equation 3. The anode reaction for further extraction of vanadium from VC0.88 is
VCo.88 + 2 Cl- → VCl2 + 0.88 C + 2 e-………………………………………………..(9)
and the net cell reaction is identical to equation 4.
Nowotny and Nickel stated that the electrical conductivities of the refractory carbides are comparable to those of the corresponding metals. The high melting point of V2C, 2,438° K, makes it suitable as a solid cell feed. The inert-atmosphere, molten-salt electrolytic process has been shown to be an effective method for reducing the interstitial content of vanadium. Considering these factors, the preparation of vanadium by electrolysis of V2C in a molten-salt electrolyte appeared feasible.
Equipment and Procedure
Helium-atmosphere electrolytic cells 6 and 12 inches in diameter were used in this investigation. Figure 2 shows a schematic drawing of a cell. The cell consisted of a Hastelloy N electrolyte chamber equipped with a graphite liner encased in a molybdenum liner, a water-cooled air lock equipped with vacuum and helium connections, and a water-cooled slide valve which provided an airtight seal between the chamber and the air lock. The cell chamber was heated with a resistance furnace.
Vanadium carbide used in this investigation was in the form of 1-inch-diameter by ½-inch-thick pellets supplied by Union Carbide Corp. After crushing into ¼- to ½-inch chunks, the vanadium carbide was charged between either a porous Alundum tube having an approximate particle retention
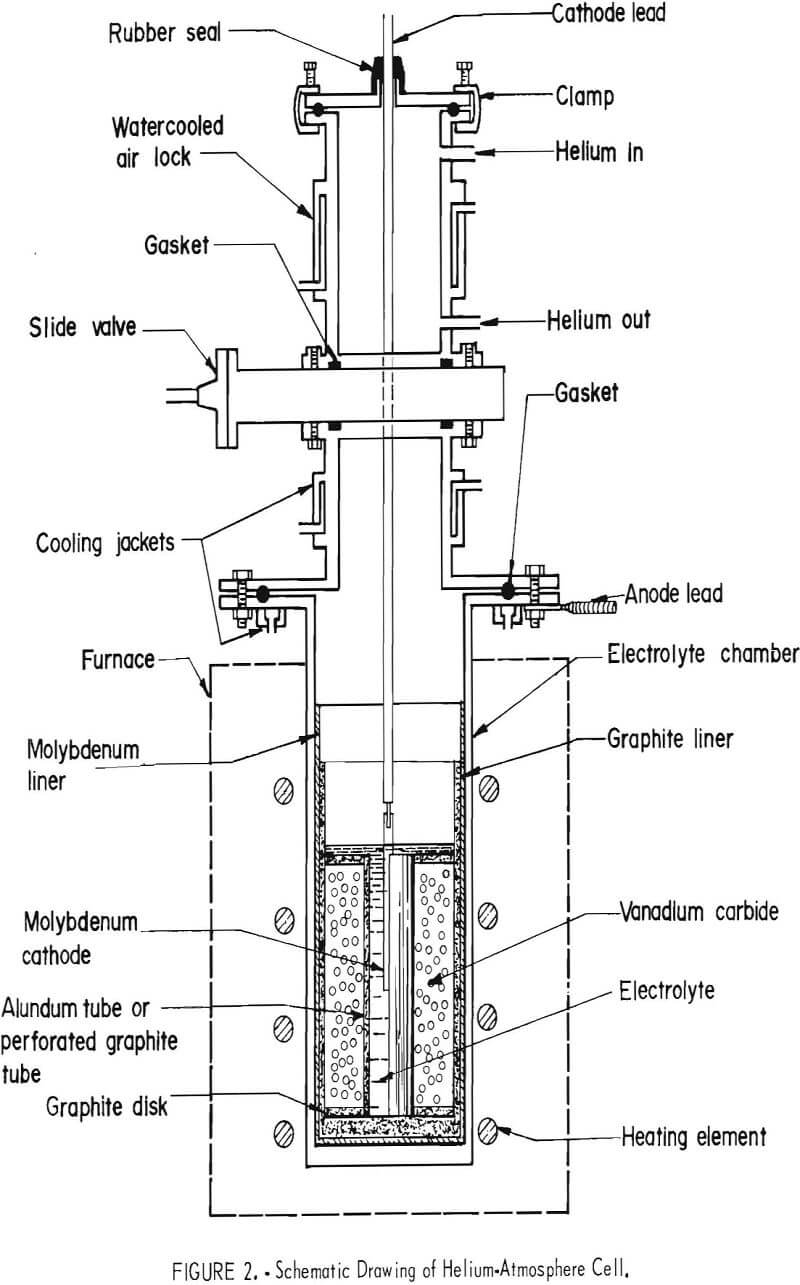
of 20 microns, or a perforated graphite tube, and the crucible side wall. The tube’s function was to isolate the carbide from the cathode compartment. When a perforated graphite tube was used, it was wrapped with an 80-mesh molybdenum screen, as shown in figure 3 to prevent the entry of vanadium carbide or free carbon produced in electrolysis into the cathode compartment. Figure 4 shows V2C charged into a 12-inch-diameter cell between the graphite crucible and the perforated graphite tube.
The electrolyte was composed of 5 to 12 percent vanadium dichloride dissolved in a mixture of 48 weight-percent barium chloride (BaCl2), 31 weight-percent potassium chloride (KCl), and 21 weight-percent sodium chloride. The preparation of a molten-salt mixture containing VCl2 has been previously described. The electrolyte was introduced into the cell in solid form by charging into the center of the graphite tube. It was melted under a vacuum of 0.1 atmosphere, and the chamber was then pressurized with helium, the electrolyte was frozen, and more salt was added. The melting procedure was repeated until the desired electrolyte depth was reached. A molten-electrolyte depth of 10 to 12 inches was used for a 6-inch-diameter cell; a 13- to 16-inch depth was used for a 12-inch-diameter cell. The electrolyte temperature was 670° C.
The anodic connection to the V2C anode material was through the Hastelloy crucible, the molybdenum crucible, and the graphite crucible. The cathodes were 7/16-inch- and ¾-inch-diameter molybdenum rods attached to a water-cooled copper cathode lead. In this report, a test refers to the preparation of a single deposition of vanadium on the cathode by electrolysis. A series
refers to all the tests made with a single charge of V2C anode material. The tests in a series were conducted on a continuous basis except for that time necessary to remove a deposit and insert a new cathode, or to add more electrolyte to compensate for electrolyte dragout. When a test was completed, the deposit was withdrawn from the electrolyte and cooled to room temperature (25° C) in the air lock. The slide valve was closed and locked to protect the inert atmosphere over the electrolyte before the deposit was removed. A new cathode was attached to the cathode lead and inserted in the air lock, which was then evacuated and flushed with helium before reopening the slide valve and lowering the cathode into the electrolyte.
The deposit was leached overnight by immersion in a 2-percent hydrochloric acid (HCl) solution to dissolve most of the entrapped electrolyte. The vanadium crystals were then stripped from the cathode rod, washed with distilled water, rinsed with acetone, and dried. The cathode product was sized by screening into plus and minus 80-mesh fractions.
Experimental Work
An exploratory study was first conducted to determine the conditions necessary for the electrolytic extraction of vanadium from V2C. The results showed that vanadium could be extracted from V2C and recovered as metal at the cathode. Deposition occurred with an applied cell potential of 1.00 volt, which corresponded to a net potential of 0.2 volt, and an initial cathode cur¬rent density of 50 amp/ft² in an electrolyte containing from 4 to 10 percent vanadium dichloride. Iron and chromium present as impurities in the V2C were also extracted, which resulted in contamination of the vanadium product. The use of low cathode current densities (<100 amp/ft²) resulted in a higher proportion of metallic impurities in the vanadium.
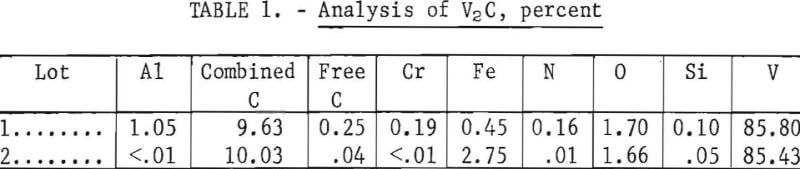
Three series of tests were then made using two different lots of V2C. The tests were all made using BaCl2-KCl-NaCl-VCl2 electrolytes at 670° C. The quality of the V2C is shown in table 1. Oxygen was the major impurity with lesser amounts of aluminum, iron, and chromium in lot 1 V2C. Iron and oxygen were the major impurities in lot 2 V2C. In addition to the analyses shown, the carbides contained up to a total of 0.5 percent other metallic impurities. X-ray diffraction patterns on both lots identified V2C as the major component in the carbide.
The first series of tests was made in a 6-inch-diameter cell using lot 1 V2C as the anode material. This series consisted of 30 depositions made with cell potentials of 0.4 to 0.8 volt, currents of 10 to 20 amperes, and initial cathode current densities of 200 to 400 amp/ft². A total of 1.6 kg of vanadium was prepared from 2.3 kg of V2C in 2,100 ampere-hours of electrolysis. Seventy-five percent of the product was plus 80-mesh vanadium, and 25 percent was minus 80-mesh vanadium.
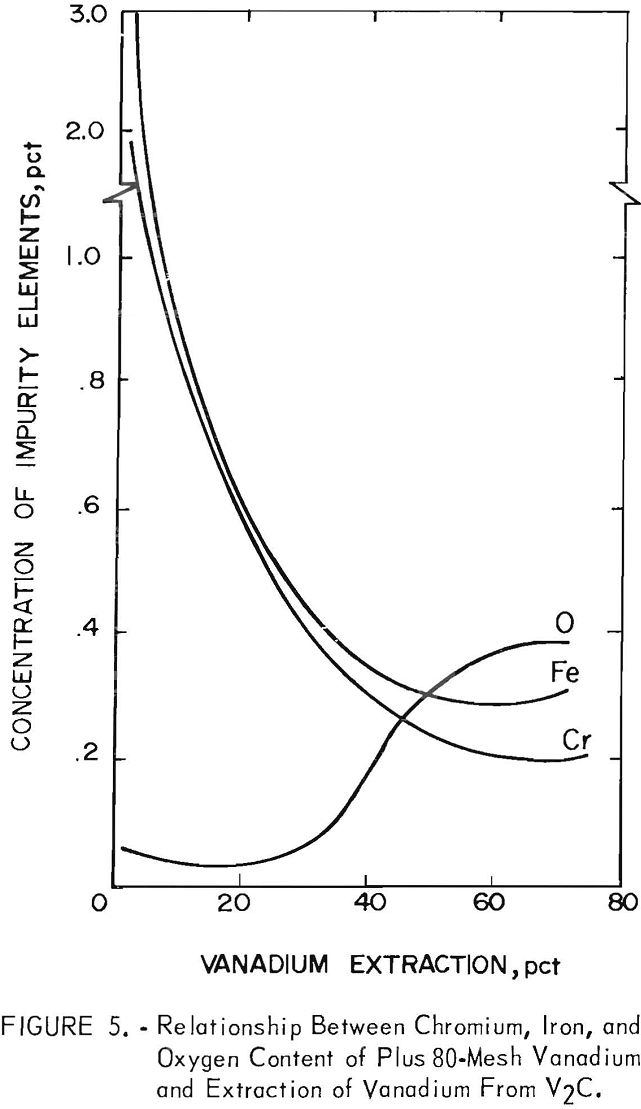
The contamination of the first series plus 80-mesh vanadium with chromium, iron, and oxygen was determined as a function of the vanadium extracted. This relationship is shown in figure 5. The contamination of vanadium with chromium and iron was highest during the first 30 percent of vanadium extraction. The oxygen content of the first 35 percent vanadium extracted was low, but increased with increasing depletion of the anode. The hardness of vanadium is directly proportional to its oxygen content, but is not appreciably affected by small amounts of metallic impurities. A ductile, low-oxygen vanadium product was prepared during the first 35 percent extraction of vanadium. Unfortunately, the transference of chromium and iron from the V2C reduced the possibility of this product being both high-purity and ductile vanadium. The contamination of the minus 80-mesh vanadium by these three elements was similar to that of the plus 80-mesh vanadium, but the impurities were in higher percentages.
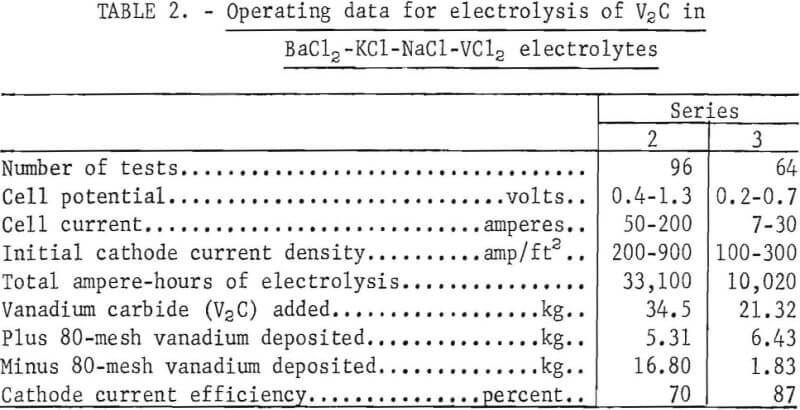
The second series using lot 1 V2C and the third series using lot 2 V2C were made in 12-inch-diameter cells. In each of these series, the tests were divided into a conditioning and a regular electrolysis. The purpose of the conditioning electrolysis was to remove the bulk of the metallic impurities before the regular electrolysis. It was made at initial cathode-current densities of 50 to 80 amp/ft². Each series was terminated when the oxygen content of the minus 80-mesh product increased to 1 percent. The operating data for the second and third series of regular tests are presented in table 2.
In the second series, 3.36 kg of vanadium containing an average of 2.0 percent metallic impurities was prepared in 4,000 ampere-hours of conditioning electrolyses with lot 1 V2C. The electroextraction was then continued with initial cathode current densities ranging from 200 to 900 amp/ft². After another 4.8 kg of vanadium had been extracted, the deposits deteriorated with respect to the yield of plus 80-mesh vanadium and to increased electrolyte dragout. A total of 22.11 kg of vanadium was produced, 24 percent of which was plus 80-mesh vanadium and 76 percent of which was minus 80-mesh vanadium. The metal-to-electrolyte dragout ratio was 0.4 to 1.0 for the deposits.
The V2C used in the third series contained more iron and required 7,000 ampere-hours of conditioning electrolysis to produce 5.99 kg of vanadium containing an average of 2.0 percent iron. The electroextraction was then continued with initial cathode current densities ranging from 100 to 300 amp/ft² to produce 8.26 kg of vanadium. The latter contained 77 percent plus 80-mesh and 23 percent minus 80-mesh vanadium. The metal-to-electrolyte dragout ratio was 0.8 to 1.0 for the deposits.
Analysis of electrolyte samples from all of the series indicated that the valence of the vanadium in the electrolyte ranged from 2.00 to 2.20 during the extraction process. The variation indicated the occurrence of oxidation of vanadium dichloride to trichloride and its subsequent regeneration by the reaction between the trichloride and the vanadium carbide in the anode area during electrolyses. The cathode current efficiencies were computed on the basis of the reduction of divalent vanadium.
In the second series of tests, after approximately 53 percent of the vanadium had been extracted, a sample of the anode material was removed for examination. Both X-ray identification and chemical analysis of the anode showed that it was VC. Thus, after 50-percent extraction of vanadium from V2C, the anode material had changed from V2C to a VC phase, as postulated in equation 3.
Discussion of Results
Influence of Cathode Current Density
Product analyses showed that the quality of the plus 80-mesh vanadium was better than that of the minus 80-mesh vanadium in the deposits produced by electrolyzing V2C anode. Operating parameters suitable for producing deposits that would consist of more of the coarse metal product were determined by comparing the yield of plus 80-mesh vanadium produced in the first and second series of tests. The relationship between the plus 80-mesh vanadium production and the vanadium extraction from V2C in each series is represented by the graph shown in figure 6. The yield of plus 80-mesh vanadium decreased with the increasing extraction of vanadium from the V2C. The production of the plus 80-mesh vanadium began to decrease in the first series when the vanadium extraction reached 50 percent in the anode. The production of plus 80-mesh vanadium dropped much earlier in the second
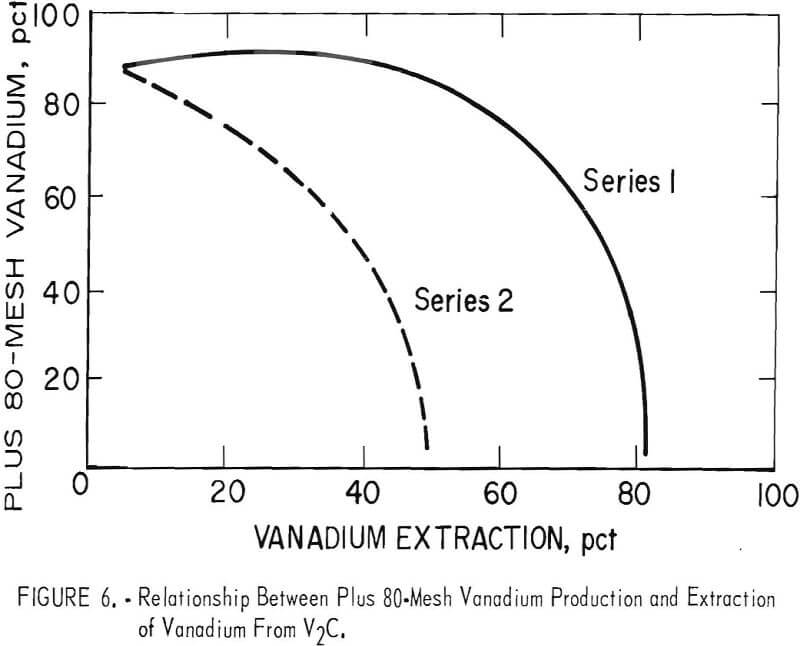
series and decreased to only 4 percent when 50-percent extraction was attained. The most significant measurable difference between electrolyses in the first and second series was the applied current; therefore, electrolysis of V2C with a high current or current density resulted in a lower yield of plus 80-mesh vanadium. By the use of lower cathode current densities in the third series electrolyses, yields of plus 80-mesh vanadium were obtained comparable to those in series 1.
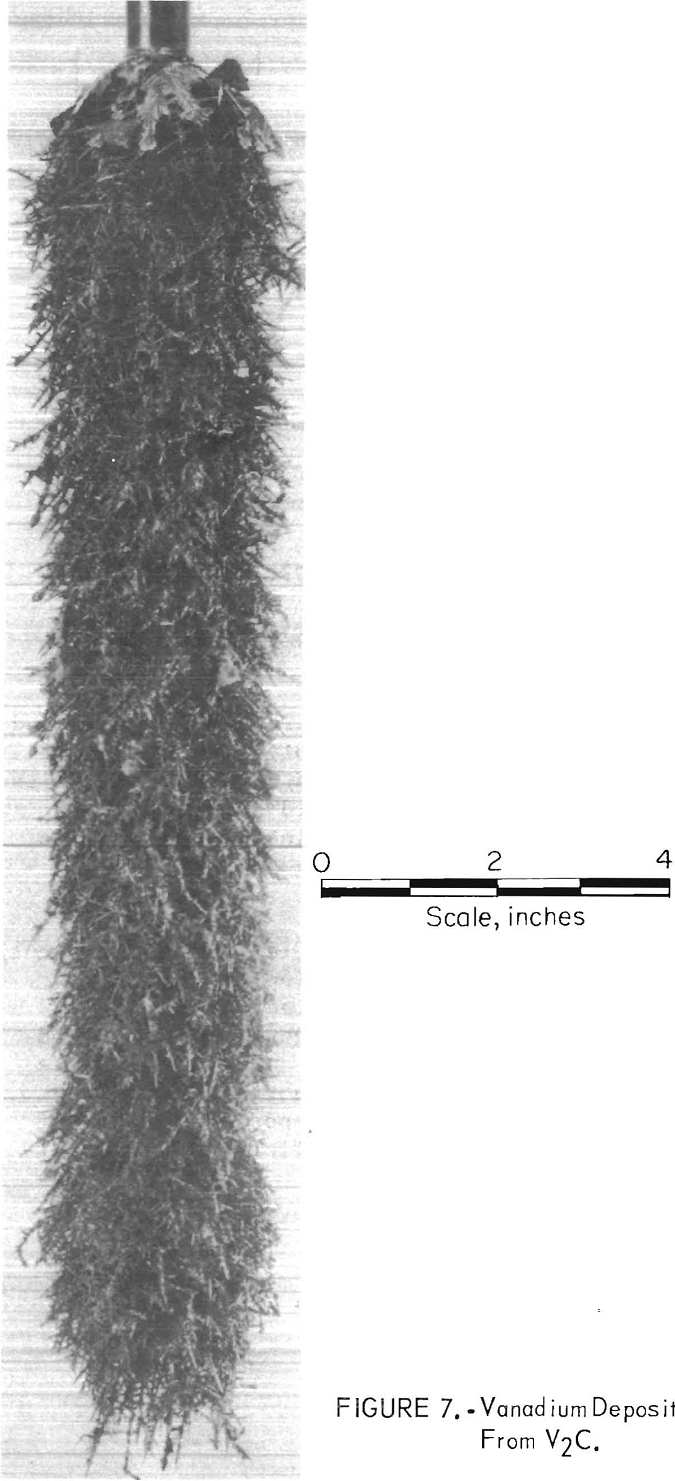
In all three tests, when the vanadium extraction was less than 20 percent, electrolyses with cathode current densities ranging from 250 to 470 amp/ft² resulted in higher yields of plus 80-mesh vanadium, low electrolyte dragout with deposits, and good cathode current efficiencies. Figure 7 shows a good- quality deposit from the second series. It was made with an initial cathode current density of 450 amp/ft² for 200 ampere-hours of electrolysis with 15-percent vanadium extraction from the V2C anode. The deposit consisted of 83 percent plus 80-mesh vanadium. The cathode current efficiency was 86 percent for the test.
Quality of the Vanadium Product
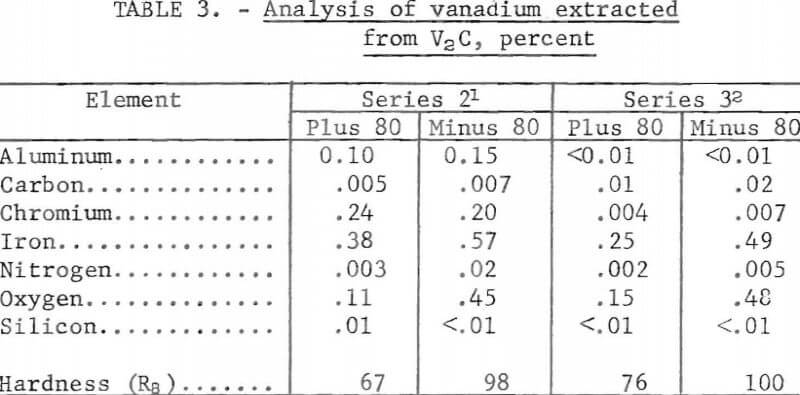
Table 3 shows typical analyses of vanadium produced in the second and third series,. The plus 80-mesh vanadium produced in the second series was 99.2 percent whereas the minus 80-mesh metal was 98.6 percent vanadium. Both fractions of the products contained aluminum, chromium, iron, and oxygen as the chief impurities; with the exception of chromium, their concentration was higher in the minus 80-mesh vanadium. The lack of metallic impurities other than iron in lot 2 V2C resulted in a better grade vanadium product in the third series. The plus 80-mesh metal contained 99.6 percent vanadium and the minus 80-mesh metal, 99.0 percent vanadium. The chief impurity elements in the product were iron and oxygen.
The product from the conditioning electrolyses in each series of tests was ductile vanadium containing approximately 2 percent metallic impurities. The interstitial impurity levels in the products were low; for example, analysis of products from the second series showed oxygen contents ranging from 0.04 to 0.12 percent, nitrogen from 0.002 to 0.007 percent, and carbon from 0.01 to 0.04 percent. The average hardness of all the products was 78 Rockwell B, which is well below the hardness value of nonductile vanadium.
The success in producing ductile vanadium with over 99 percent purity by electroextraction of V2C will depend largely on the use of V2C with low metallic impurities. Iron and chromium are metallic elements that must be kept to a minimum for successful production of ductile vanadium, without resorting to a conditioning electrolysis to remove the bulk of these elements.
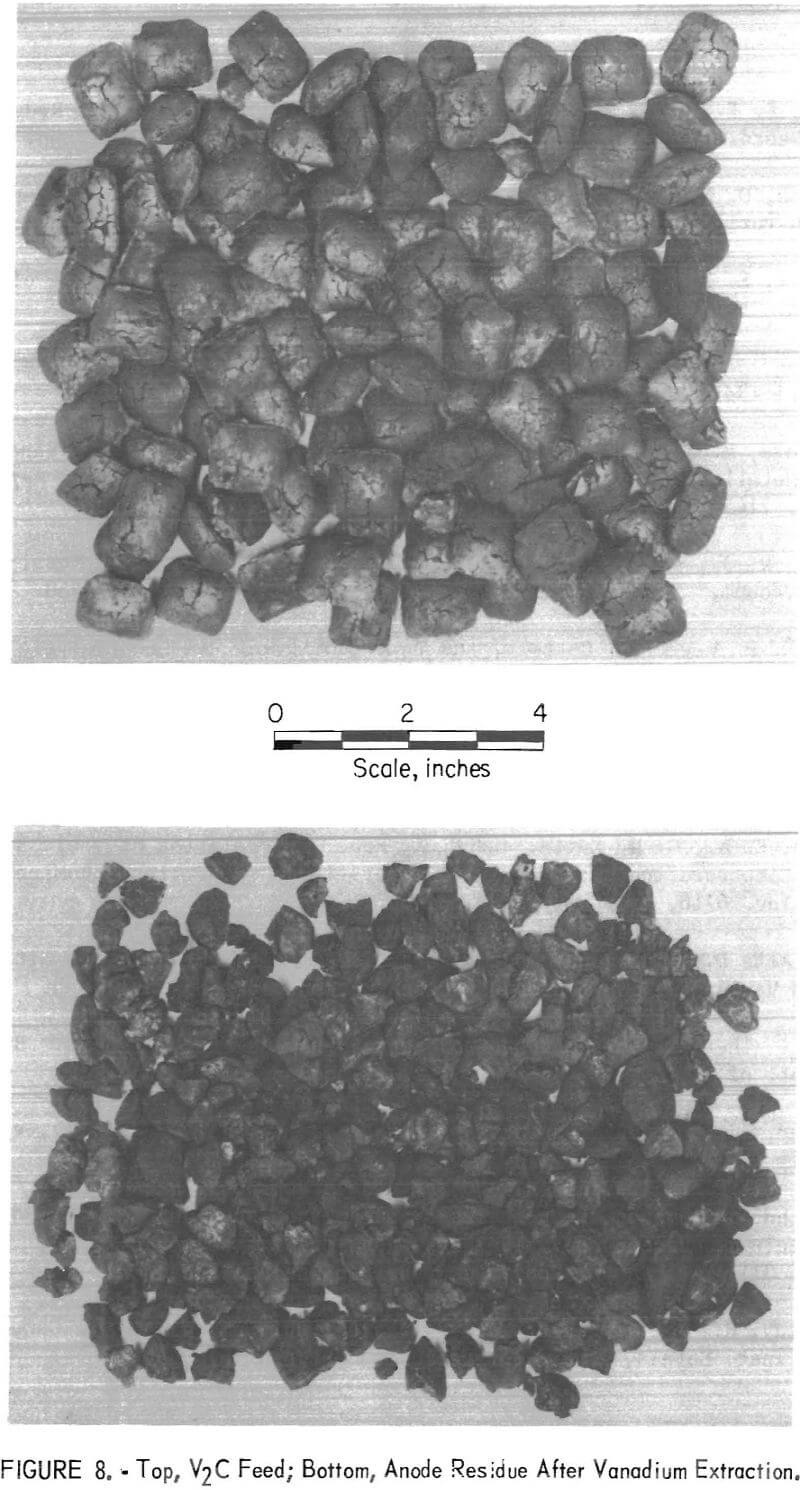
The carbon and oxygen contents of the V2C remained essentially at the anode, while the vanadium was extracted and deposited at the cathode. Extraction of the vanadium from the vanadium carbide left a skeleton of carbon in approximately the same shape as the original V2C. The top of figure 8 shows the V2C anode feed, and the bottom shows the residue after extraction. The concentration of carbon and oxygen at the anode is exemplified by examination of the anode residues left after the completion of the third series of tests. Analysis of the residue showed that it contained, in percent, 58.1 vanadium, 8.6 combined carbon, 18.9 free carbon, 7.3 oxygen, 1.4 chlorine, 0.5 barium, and 0.2 iron. X-ray examination of the anode residue indicated the presence of VC0.7 and VO0.2 in the material.
Vanadium Recovery
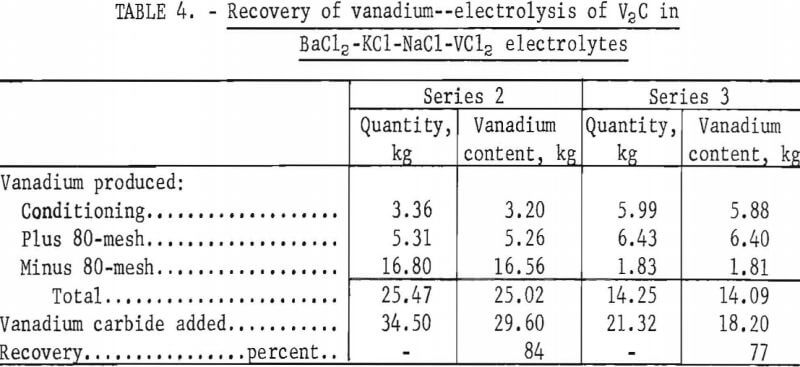
The vanadium recovery at the cathode in series 2 and 3 is shown in table 4. The total extraction of vanadium was 84 percent in series 2 and 77 percent in series 3. The overall recovery of vanadium in both series averaged 81 percent and indicated that extraction of vanadium also occurred from the VC0.88 phase of the anode. The recovery of vanadium was limited by the rapid increase in impurity content of the vanadium product after approximately 80 percent extraction, as shown in figure 5. Both series of tests were terminated when the oxygen content of the minus 80-mesh portion of the products reached 1 percent.
Electrorefining of the Vanadium Products
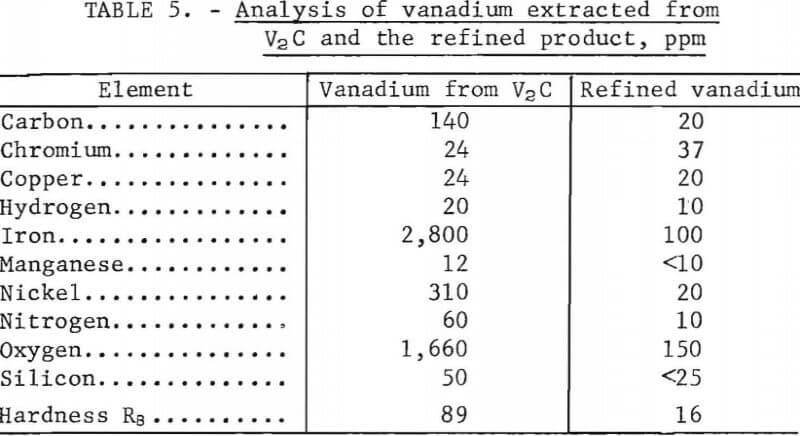
The vanadium produced from V2C in the third series of tests was further purified by electrorefining to produce high-purity vanadium. Molten-salt electrorefining of a mixture of 4.54 kg of plus 80-mesh vanadium and 1.10 kg of minus 80-mesh vanadium in a KCl -LiCl-VCl2 electrolyte at 600° C (8) produced 4.03 kg of high-purity metal. The cathode current density used for refining was 200 to 300 amp/ft². Analyses of the feed material and refined product are shown in table 5. The refined product was high-purity (99.96 percent) vanadium.
Conclusions
The preparation of vanadium from a V2C-type vanadium carbide was demonstrated by molten-salt electrolyses in helium-atmosphere cells. An electrolytic process was developed for obtaining ductile vanadium metal from commercial V2C carbide in a BaCl2-KCl-NaCl-VCl2 electrolyte at 670° C. The recovery of vanadium at the cathode ranged from 77 to 84 percent. The products consisted of vanadium with purity ranging from 98 to 99.6 percent and with hardness ranging from 67 to 100 Rockwell B. Metallic impurities in the carbide such as chromium and iron contaminated the cathode product. The vanadium extracted from the V2C was an excellent cell feed for preparing high-purity vanadium in an electrorefining process.