Table of Contents
The recovery of silver and gallium from flue dust with acidified thiourea solutions was examined. The studied parameters were temperature, particle size, thiourea concentration, acid concentration, and the presence of oxidants. The study of the extraction of these two metals was undertaken in sulfuric acid medium. Silver dissolution was dependent on all examined parameters, whereas gallium extraction was only dependent on the initial acid concentration.
An important source of gallium is the flue dust or fly ash generated from various industrial operations, such as the production of producer gas from coal, the smelting of copper or zinc ores, and the production of elemental phosphorous from phosphate rock. The ash from the electrothermic reduction of phosphate rock typically contains about 0.4 kg/t of gallium. However, the dust from some English coals contains as much as 0.4% gallium and may reach as high as 1.6%.
Experimental
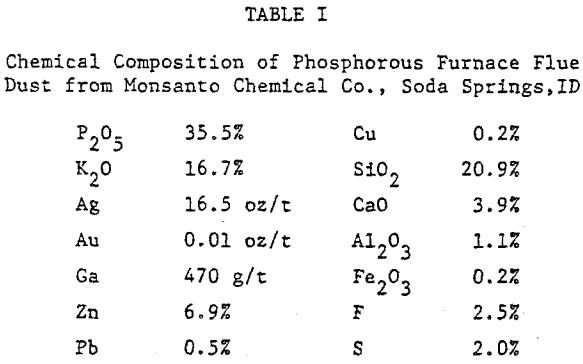
For the present work, flue dust obtained from the Monsanto Chemical Company’s elemental phosphorous plant at Soda Springs, Idaho was studied. The dust was dry sieved as received into monosized fractions in the range +28 to -325 mesh. Each size fraction was analyzed for silver and gallium, silver by fire assay and gallium by a concentrated nitric/sulfuric acid leach followed by atomic absorption.
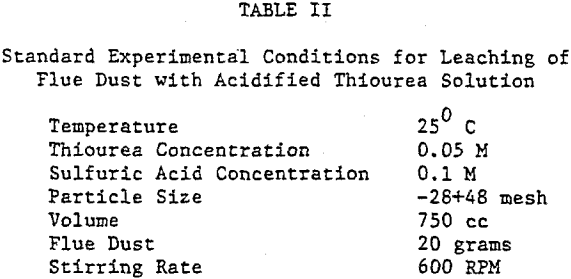
Results
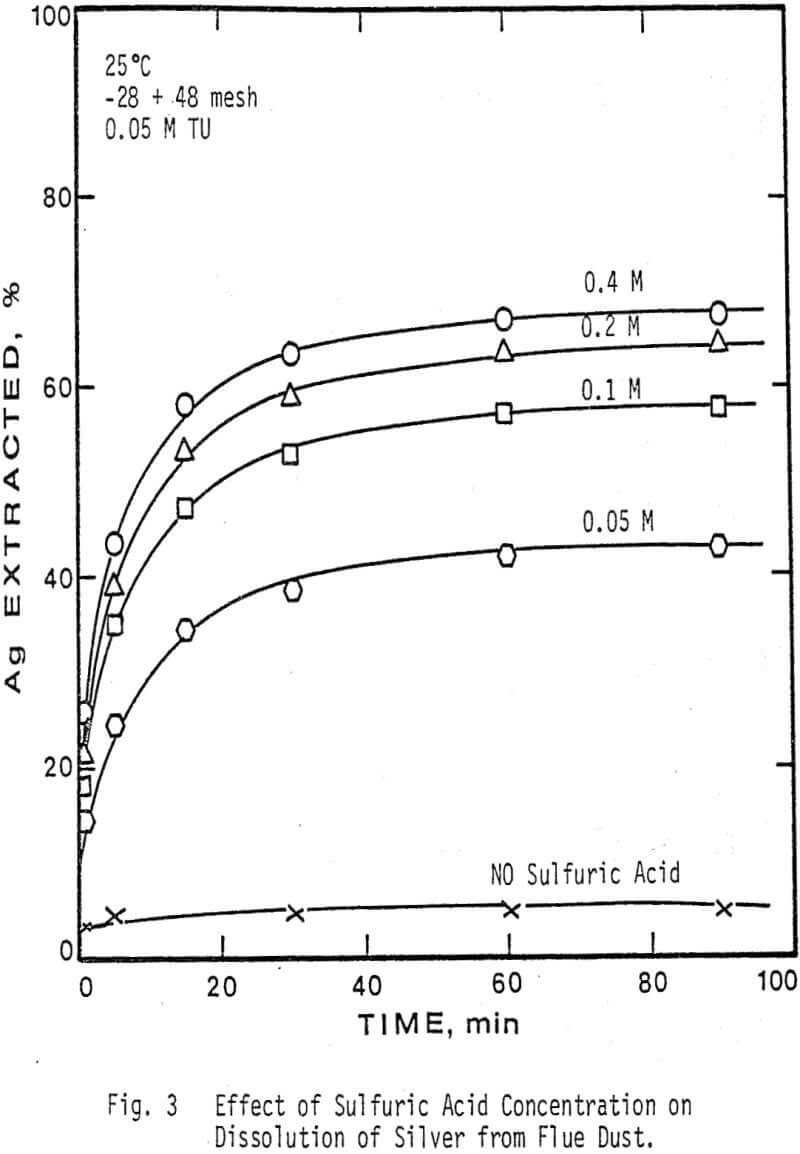
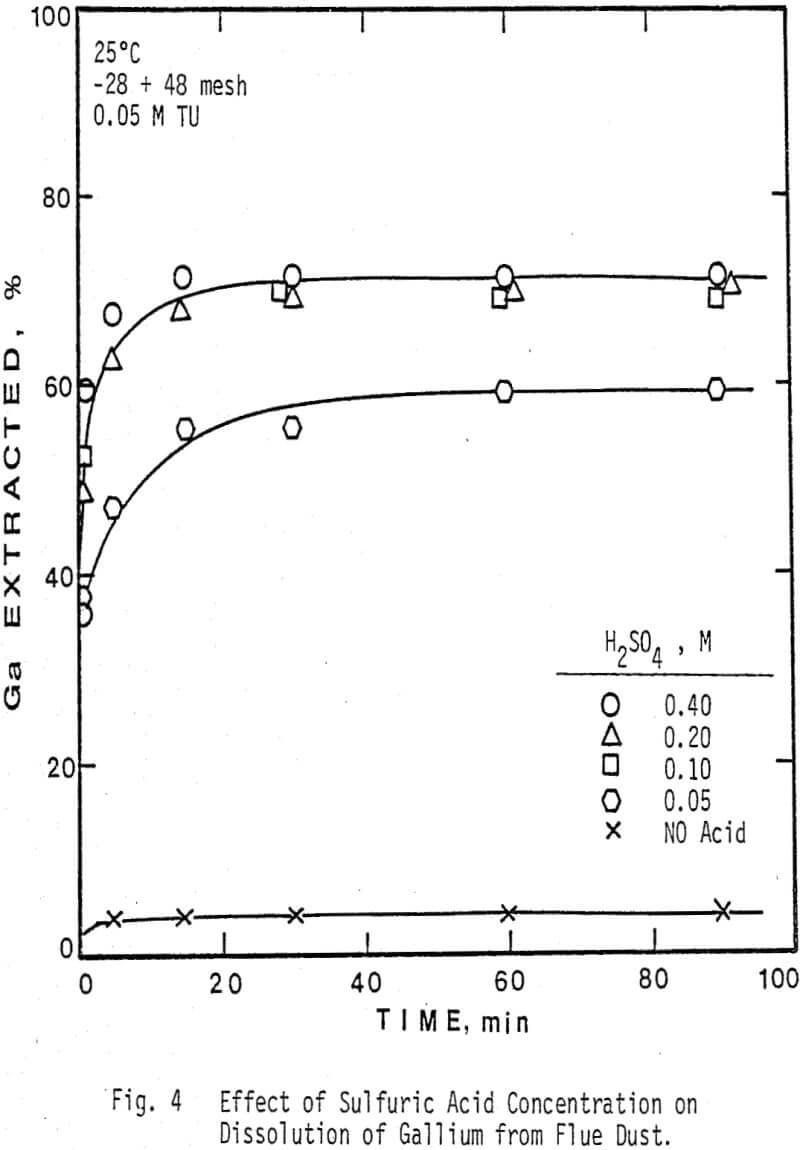
The extraction of silver and gallium from the flue dust was examined as a function of four parameters: Thiourea concentration, sulfuric acid concentration, particle size, and temperature. The effect of several oxidants on the dissolution rate of silver and gallium was also examined.
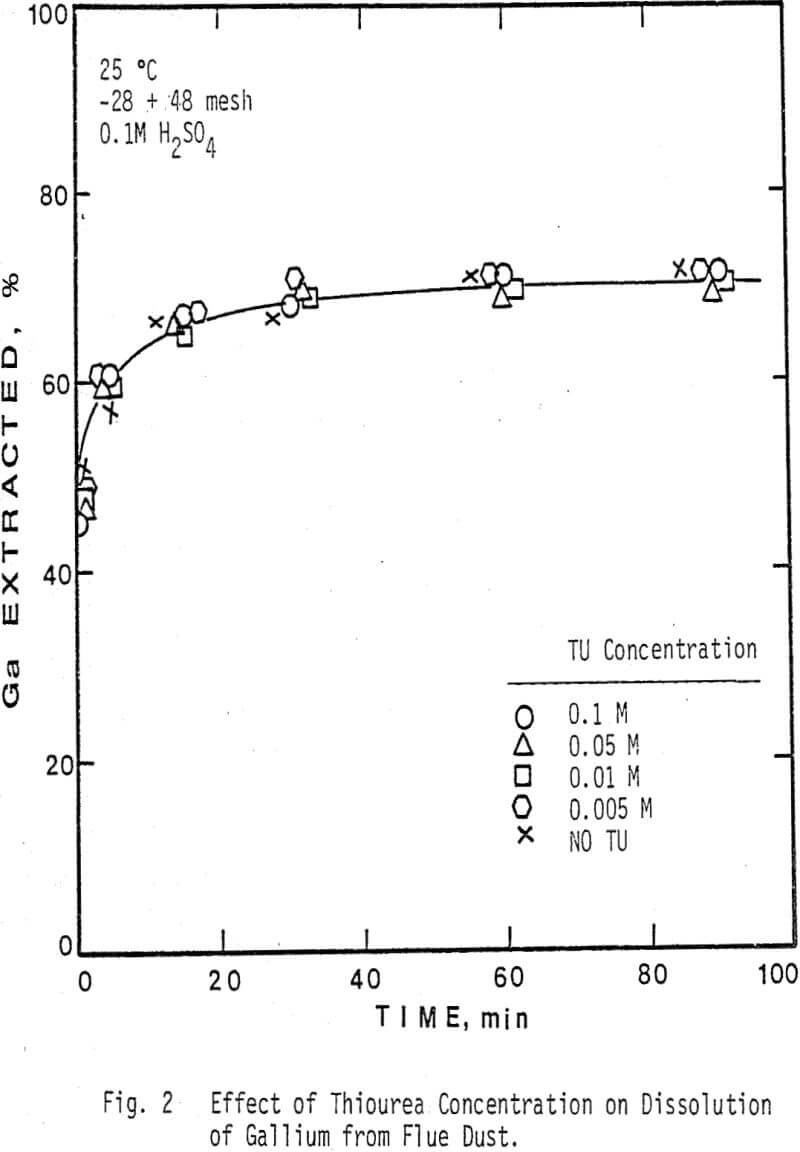
The thiourea concentration range studied for the extraction of silver and gallium from the dust was 0-0.2M. It can be seen that the leaching of silver from the dust is highly dependent on thiourea concentration, whereas the rate of extraction of gallium is independent of thiourea concentration. Note that the initial rate of extraction of gallium is higher than that of silver & that the maximum amount of gallium extracted from solution (70%) is dissolved in 15-20 min.
The sizes studied ranged from -150+325 mesh to -28+48 mesh. It can be seen from both figures that the dissolution of both elements is essentially independent of size, although there is a small increase in the rate of dissolution of gallium from the smaller sizes. These results would be important in the development of a leaching process because the dust is predominately -28 mesh and could be leached as received, thus realizing a considerable savings in energy.
Temperatures greater than 45 °C were not examined due to the instability of thiourea at high temperatures in solution. Note that silver extraction is dependent on the temperature, whereas gallium dissolution is nearly independent of the temperature chosen.
The oxidants examined were oxygen, hydrogen peroxide (H2O2), and ferric ion. The rate of recovery of silver is dependent upon the quantity of oxidant present. As the amount of oxidant is increased, the rate of silver recovery and amount extracted both show an increase, maximum dissolution being obtained with 0.006 M Fe+³ in 30 minutes. However, addition of an excess of oxidant will decompose thiourea and cover the particles in solution with a fine coating of sulfur, thus leading to lower recovery of precious metal.
Since the exact mineralogical composition of the dust is not known at this time, the precise chemical reactions occurring in this leaching system would be difficult to predict. Approximately 60% of the dust consists of silica and various complex phosphates. The metallic elements present may be in the form of their corresponding silicates and/or phosphates, or in the case of several metals, in their oxide form.
The purpose of the oxidizing agent is to oxidize thiourea to formamidine disulfide, which is the compound thought to be responsible for the actual dissolution and complexation of the silver present.
The activation energy for silver in this system is low, about 3.9 kcal/mole. This result would tend to support a mass transport controlled reaction, i.e., diffusion of thiourea into the pores of the dust. The activation energy for gallium was found to be 1.5 kcal/mole, about 2.5 kcal/mole less than the activation energy for silver dissolution. The rate of gallium extraction is much higher than that of silver.
Thiourea is first oxidized to formamidine disulfide, which can then be reduced back to two molecules of thiourea, or break down to eventually give cyanamide and sulfur. Formamidine disulfide is necessary for the successful extraction of silver into solution, whereas the formation of cyanamide and sulfur lead to losses of reagent.
In view of the excellent results obtained by using acidified thiourea solution to extract silver and gallium, it was decided to develop a scheme or proposed flow diagram for the recovery of silver and gallium. Carbon adsorption of silver from acidified thiourea solutions would represent the most logical approach for recovery of silver from leach solutions. With these results in mind, a proposal for a fully integrated system for the recovery of silver and gallium was developed. The description of this system is as follows:
The flue dust (sized to pass 28 mesh) is first leached with hydrochloric acid in order to extract gallium as the chloride. The chloride solution would be more amenable to recovery by ion exchange in the next step, and the acid would remove most of the phosphates and zinc present in the dust.
After filtration, the solution is passed through an ion exchange column to remove gallium. The residue from the HCl leach is then leached with acidic thiourea solution and +28 mesh carbon (carbon in leach). After filtration, the carbon would be recycled to the main reactor and the pregnant solution would then be sent to cells for electrowinning of silver.