Primary smelting of zinc in horizontal retort distillation furnaces leaves a residue consisting of the following: unburned carbon, iron as metal shot and stringers, copper inclusions in the iron as metal and as sulfides, silver in solid solution in the copper, and glassy slag. After removal from the retorts and cooling, the residue is crushed and magnetically separated to produce an upgraded copper-silver material that will economically justify shipment to a copper smelter for the recovery of these two metals. If the magnetic separation fails to achieve necessary enrichment, the residue is stockpiled. When the product is salable, payment is for copper and silver only, zinc and iron being lost as they are not recovered by the copper smelter. An estimated 200,000 tons of magnetic residues are generated annually in the United States. This estimate is based on the production of slab zinc as shown in the Minerals Yearbook. In addition, sizable tonnages of the residue have accumulated over the past 40 to 50 years, the operating life of most of the horizontal retort smelters. One Midwest plant reports an estimated 200,000 tons of residue in storage at its plant in 1972.
Based on prices quoted in August 1972, the potential worth of the contained zinc, copper, silver, and iron in an average residue are as shown in table 1.
Because copper smelterss the only significant consumer of the enriched residue, are becoming more reluctant to purchase the material, the development of technology for alternative means of recovering the metal values is increasingly important. Accordingly, the objective of the work undertaken by the Bureau of Mines was to demonstrate the technical feasibility of recovering the metals in useful form.
The Residue
The material described in the following paragraphs is considered representative of the magnetic fraction of the furnace residue produced by a mid-western plant at an average rate of about 40 tons per day. A partial analysis of this magnetic fraction is given in table 2.
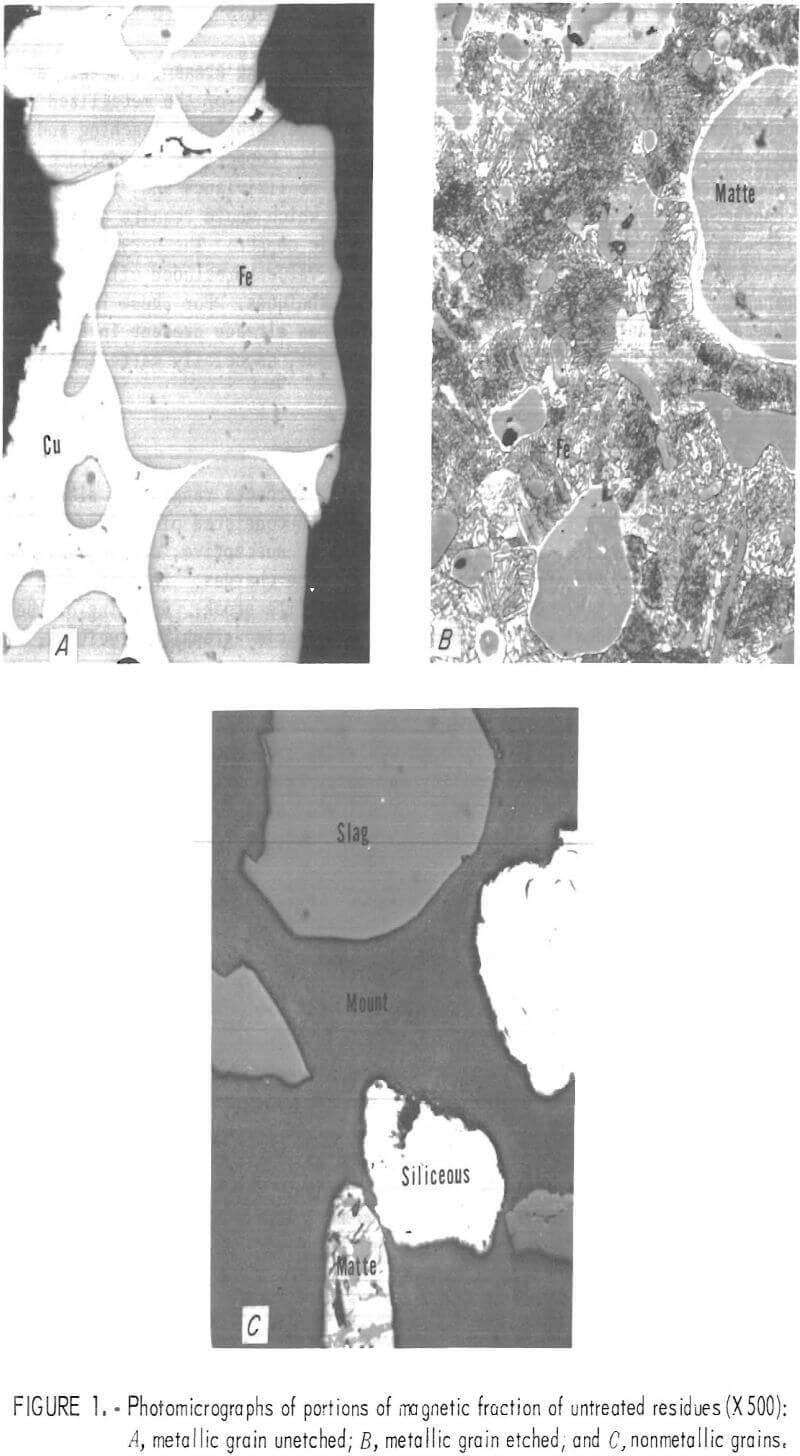
The complex structure of the magnetic material, which includes unburned carbon, metallic iron, metallic and sulfide copper, silver, and slag, is illustrated in figure 1 which consists of three photomicrographs of specific areas of the residue and includes both metallic and nonmetallic grains. Magnification is X 500 in all instances. Figure 1A represents an unetched grain of metal and shows the mutual encapsulation of iron and copper; figure 1B is of metal etched with Nital and shows minute grains of sulfide in metallic copper which in turn is surrounded by a matrix of iron; figure 1C shows glassy slag or the nonmetallic part of the residue.
Experimental Procedures and Results
Exploratory studies to delineate the most promising areas for detailed research included chemical leaching, physical separation of the metallized constituents, and pyrometallurgical treatments The first two, leaching and physical separation, were found to be impractical. Copper was encapsulated to such extent in the iron as to be unavailable to lixiviants and, in addition, an appreciable portion was present as sulfides, which would require entirely different solvents from those useful for metallic copper. The very finely divided state of a major part of the metallized values precluded effective separation from the slag by mineral dressing techniques. For these reasons and because an appreciable quantity of sulfide was already present in the residue, it was decided that pyrometallurgical methods, especially matte smelting, would be most appropriate.
Pyrometallurgy
All work in this area was done in induction furnaces ranging in size from 50- to 150-lb capacity. Because the charges which consisted of residue, sulfidizer, and reductant, were not electromagnetically susceptive, carbon, silicon carbide, or similar crucibles were used to melt the charges. In most cases the crucibles were machined from high-purity graphite stock. Refining of the iron to recover residual copper was carried out in clay-graphite crucibles.
Initial studies determined the most desirable reductant, sulfidizer, and temperature. From reductants such as charcoal, coke, and waste granular graphite, coke was chosen although each was effective, and final choice, in practice, would depend on availability and cost. Elemental sulfur, pyrite, sphalerite, and hydrogen sulfide were investigated as sulfidizers; pyrite was the most efficient. Each will be discussed in subsequent passages.
Operating temperatures ranged from 1,400° to 1,700° C, the higher level being required for complete reaction. A temperature of 1,400° C melts the charge and suffices to eliminate essentially all of the zinc as fume. No attempt was made to recover the zinc as metal, rather it was allowed to oxidize and the oxide was recovered in a wet scrubber. The high temperature attained at the end of the reaction completely eliminated zinc from the slag, metal, and matte, and for this reason zinc is not accounted for in tabulated data. The final temperature of 1,700° C the necessity for which will be discussed in subsequent paragraphs, is easily attained through the energetic oxidation of zinc and CO coupled with a small increase in power input. Only a short holding time is required at this temperature. Temperature readings were obtained by sighting an optical pyrometer down a closed end, graphite tube immersed in the melt with the closed end in the metal. Readings were thus unhindered by fume and surface scum.
The quantity of matte formed was directly related to the amount of sulfidizer. With no addition, a small matte formed from sulfur present in the residue; at the other extreme, all of the iron and copper could be sulfidized.
As the research progressed, it became apparent that a major problem was that of residual copper in the iron. Under all conditions in which iron was reduced to metal, the metal contained a minimum of about 2.5 percent Cu. This represented an unacceptably high loss of copper and, in addition, rendered the iron unfit for foundry iron. Soluble at the temperature of molten iron, upon cooling this copper precipitated out as dendrites in an iron matrix. To render the iron product salable, two options were available: (1) Increase the copper content to the maximum possible, then granulate for possible use as cementation medium, or (2) remove the copper from the iron to an acceptable degree (0.2 pct Cu).
Granulation
A use for the copper-bearing iron that would permit consumption of both metals is that of cementation as practiced by the copper mining industry. For this option, the copper content of the iron was maximized by smelting under reducing conditions with no addition of sulfur to the charge. This yielded a small amount of high-grade matte, derived from the sulfur native to the residue, which recovered a part of the copper and practically all of the silver with the remainder reporting with the iron. This matte, of high economic worth, could be processed by known techniques. Results of such a test are summarized in table 3.

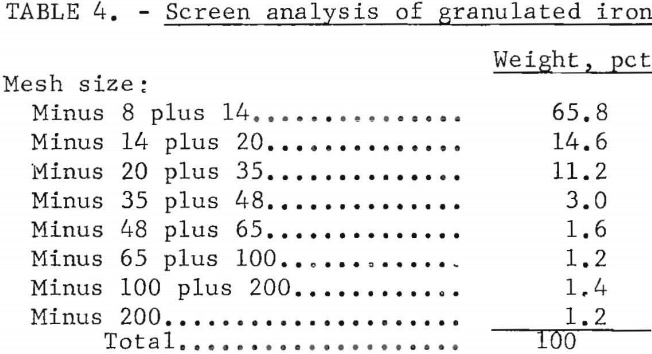
The metal, when quenched or chilled quickly, is amenable to granulation by crushing and in this form should be a satisfactory medium for the cementation of copper. Alternatively, the molten metal was shown to be amenable to shotting by pouring it into a jet of water. Qualitative tests in which copper was cemented from dilute copper sulfate solutions indicated that the crushed metal was slightly superior. In either case, copper sulfides and silver would be recovered as sludge from the cementation tanks. The size distribution of the granulated iron is given in table 4.
The production of metal shot is an established procedure and was not investigated in detail. Shotting was accomplished by pouring a thin stream of the molten copper-iron through a fan-shaped jet of water. Shot size was controlled by varying the speed (pressure) of the jet. Steam or high water temperatures were not a problem and clean, somewhat elongated shot were obtained.
Matte Smelting
In application of matte smelting to zinc retort residues, the first factor considered was the source of sulfur. Elemental sulfur, an obvious possibility, was found to be effective but its use entailed several difficulties. Subsurface injection was necessary with inherent mechanical problems. Low melting point and high vapor pressure at the temperature of the molten charge resulted in very rapid vaporization from the melt so that metal-sulfur reaction took place inefficiently; oxidation of the unreacted sulfur evolved much SO2.
Hydrogen sulfide was investigated briefly and was found to be a reasonably effective sulfidizer when lanced into the melt. However, as with elemental sulfur, there was excessive loss of unreacted gas which represented not only an economic loss but a source of pollution.
Sphalerite, an unconventional source of sulfur, was investigated briefly because it was available at the smelter and the zinc could be recovered with that contained in the residue. This mineral decomposed under the furnacing conditions previously described and the released sulfur combined with copper and iron to form a matte containing 51.9 pct Fe, 12.4 pct Cu, 0.09 pct Ag, and 33,1 pct S.
Pyrite was the most effective matte former. Thermal decomposition insured that a continuing supply of sulfur was available to the reaction. Efficient consumption of liberated sulfur was evidenced through the relatively low concentration of SO2 escaping the furnace. Exit gases entering the exhaust duct were found to contain from 65 to 200 ppm of SO2; exit gases leaving the wet scrubber, 2 to 5 ppm of SO2. Pyrite is low-cost, readily available, and its use would consume a waste byproduct of many mining operations including coal. In this case, adhering coal would be an asset. The pyrite used in this work was picked from the dumps of a western Missouri coal mining operation and contained 43.6 pct iron and 56.4 pct sulfur. The addition of flux was not required to attain sufficiently fluid slags.
Procedures
Data acquired in the final stages of the work under favorable experimental conditions are presented and discussed in the following paragraphs. In such a test, 20 lb of residue was mixed with 4 lb of pyrite and 2 lb of carbon and charged into a graphite crucible prior to heating in a 150-lb induction furnace. The carbon served not only as a reductant but prevented excessive attack on the graphite crucible. A current of 55 amperes at 650 volts (36 kW; sufficed to melt the charge and reach the desired temperature. Melting was

rapid; zinc fume appeared after about 7 minutes and fuming was essentially complete in about 22 minutes. Near the end of the zinc fuming, the temperature rose and the appearance of an intense golden-yellow flame indicated the beginning of the final reaction. The latter is exothermic as indicated by the rise in temperature, and experience showed that the reduction-sulfidization was not complete at a temperature much below 1,700° C. At this point, the slag was a light color; other wise, it was black. The influence of temperature on the removal of copper as reflected by analysis of the iron is shown in table 5.

At the end of the reaction, the melt was poured into a split conical carbon mold, and after solidification, the three phases, metal, matte, and slag, were easily separated. The metal (bottom) and matte with the slag removed are shown in figure 2. Weight distribution of the products was as follows: Metal, 5.61 lb; matte, 5.86 lb; and slag, 8.36 lb. A partial analysis of the three products is given in table 6 together with distribution of the significant elements.
It should be noted that essentially all of the silver was contained in the matte; the very small amount reporting in the metal precluded quantitative calculation of distribution. These data further show that the metal fraction would not be readily accepted as foundry iron because of the relatively high copper and sulfur content, and while the copper content represents less than 16 pct of that in the residue, its recovery would be economically desirable.
Recovery of Copper from Iron Derived from Matte Smelting
The quantity of copper contained in the iron product, about 2.5 pct, rendered it a potentially useful material for specialty alloys; however, it would not be readily accepted as foundry iron. Accordingly, decoppering of the iron was undertaken.
The three following approaches were considered: (1) Quiescent, slow cooling of the molten iron to allow coalescence or segregation of the dispersed copper and copper sulfides; (2) extraction of copper by contacting the molten iron with metallic lead ; and (3) extraction of copper from the molten iron with sodium sulfate. The difference in copper content of the samples used in these experiments, as reflected in subsequent tables, was due to the difference in degree of sulfidization of the original residue.
Quiescent, Slow Cooling
This method was investigated at the Rolla laboratories by induction melting of 10 lb of metal at 1.600° C, then pouring the metal into an insulated crucible. No additional heat was required to maintain the metal in the molten state for 30 minutes. Satisfactory separation of copper and sulfides was not achieved.
In similar but small-scale tests, conducted at College Park Metallurgy Research Center, a 54-gram sample was melted in a graphite crucible, held at 1,400° C for one-half hour, then cooled to 1,000° C using a programmed cooling rate of 2.2° C per minute. Figure 3 shows that some segregation was achieved.
Partial chemical analyses of six zones from top to bottom of the ingot are given in table 7. These results show definite concentration of copper sulfide in zone A and of metallic copper in zone B; however, the high copper content of the remaining ingot indicates that the desired reduction of this metal was not achieved.

Extraction of Copper with Metallic Lead
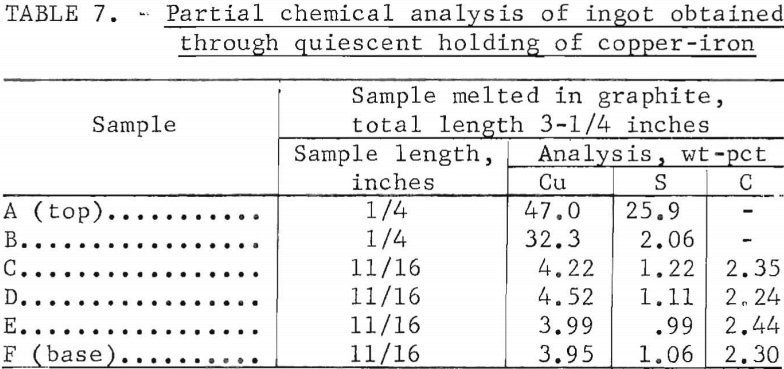
This method, based on the preferential solubility of copper in lead over that in iron at the temperature of molten iron, was investigated in detail. Two broad variables were considered: the ratio of lead to iron and the time of contact between the molten metals. The lead used in a given stage ranged from 20 to 60 wt-pct of the iron; the time of contact ranged from 1 to 10 minutes. Lead was suspended above the molten iron (-1,400° C) ; it was allowed to melt and to fall dropwise into the iron. The agitation inherent to an induction furnace provided additional contact. Pouring the melt following the addition of the lead resulted in excessive loss of lead through fuming, so the crucible was lifted from the furnace and the contents were allowed to solidify in place. The lead and iron layers were easily separated following solidification as shown in figure 4. Figure 5, a micrograph of a section of the lead ingot at a magnification of X 100, shows the extracted copper as dendrites in the matrix of lead.
An evaluation of the preceding method showed that 60 wt-pct lead with a minimum contact time, that is, removal of the crucible from the furnace immediately after the addition of the lead, resulted in the best extraction
of copper., Details of such a test using 22.7 lb of copper-bearing iron are given in table 8.
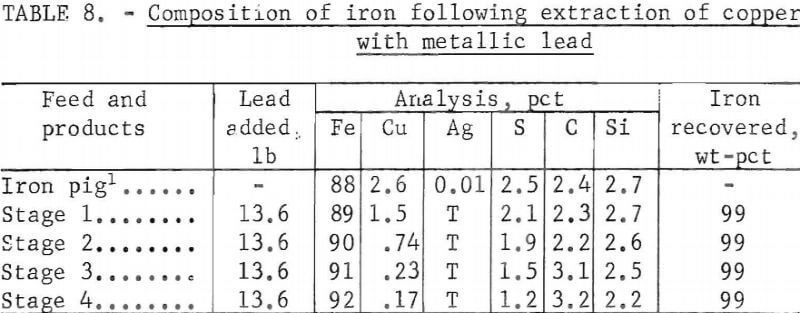
As shown, the lead was added in four successive stages, each amounting to 60 pct of the metal in the melt. It is evident that extraction of copper to an acceptable level could be accomplished in three stages. In addition, lead that is only partly loaded with copper (stage 3) would be recycled without drossing, The more heavily loaded stage 2 lead might also be returned for another cycle before drossing.
The copper-bearing lead from the test under discussion was cleaned by drossing in conventional manner. Heating to approximately 450° C with vigorous stirring served to separate the copper in the form of an easily separable dross containing 93.3 pct of the copper. Lead losses were negligible; of 13.6 lb added to the melt, 12.6 lb were recovered as clean lead for recycling, 0.9 lb was in the copper dross from which it would be eventually recovered; and the remainder, 0.1 lb, was divided between the iron and fume.
Extraction of Sulfur and Copper
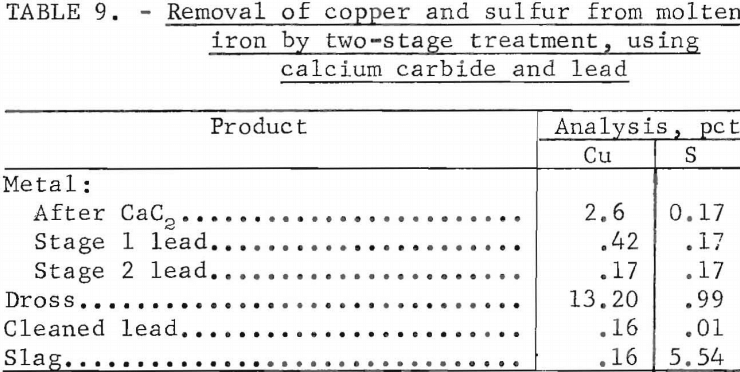
If it is advisable to remove sulfur as well as copper from the iron, it may be advantageous to utilize the following procedure: Extract the sulfur from the molten iron with calcium carbide, skim off the carbide slag, then add lead as in the preceding section. Because elemental copper is freed concomitantly with the extraction of sulfur from the copper sulfides, the copper extraction becomes more efficient. This was demonstrated by the following test in which 7.12 lb of iron was melted in an induction furnace, treated with 1 lb (14 pct) of calcium carbide, skimmed then treated twice with 4.27 lb (60 pct) of lead. This two-stage treatment produced the results shown in table 9. The lead was cleaned by drossing as described in previous paragraphs.
Extraction of Copper with Sodium Sulfate
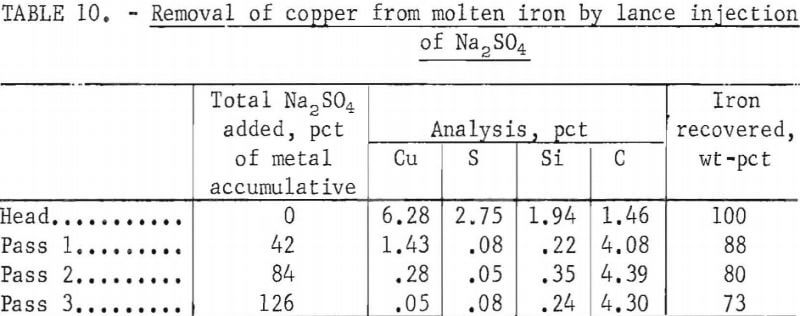
This technique for removing copper from iron was developed at the College Park Metallurgy Research Center of the Bureau of Mines and the test work reported herein was done there. It involves subsurface lancing of the molten iron with sodium sulfate to form separable sulfides, or matte. The copper-bearing iron is induction-melted in a graphite crucible and treated with Na2SO4 at 1,300° C. The sulfate was injected by lancing in three successive stages, labeled BS, CS, and DS, each addition amounting to about 42 pct of the metal charge weight. Results of such a test are shown in table 10.
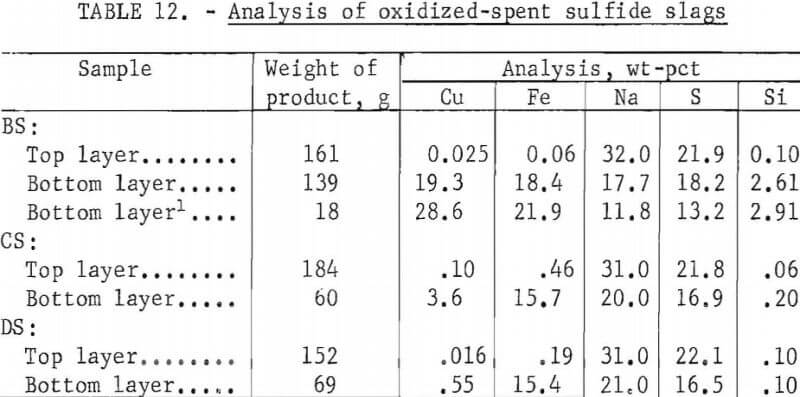
Spent sulfide slags obtained from the above refining rest were treated by oxidation to recover Na2SO4 and metal sulfide concentrate as two separate layers as shown in figure 6. Oxidation was performed by melting the slags in alumina crucibles and by lancing with air at 1,100° C to convert Na2S to Na2SO4. Copper and

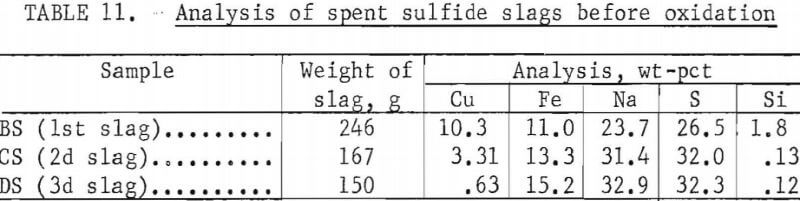
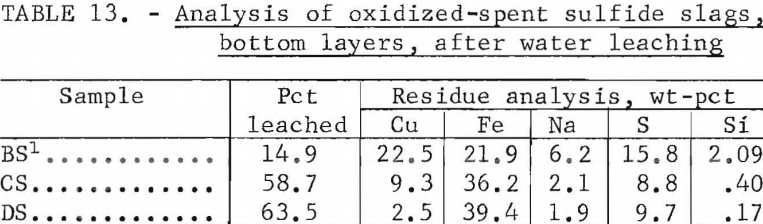
iron sulfides, insoluble in Na2SO4, precipitated as a heavy bottom layer. Analyses of spent slags before and after oxidation are shown in tables 11 and 12. Bottom layers were also leached to recover residual Na2SO4 with results shown in table 13.
Summary and Conclusions
Recovery of essentially all of the zinc, copper, silver, and iron contained in the magnetic fraction of horizontal zinc retort residues appears technologically feasible by either reduction smelting or by matte smelting
By either method, essentially all of the zinc is recovered as the oxide.
Smelting under reducing conditions with no addition of sulfur or flux yields a small quantity of rich matte and a high-copper iron. Virtually all of the silver, part of the copper, and a small amount of the iron was contained in the matte; the remainder was in the high-copper iron alloy, The alloy, when chilled rapidly, is amenable to granulation by crushing, or it may be shotted by being poured through a jet of water. These are materials that can be substituted for scrap cans in the cementation of copper from leach solutions. Copper and silver would be recovered from both matte and the consumed iron.
Matte smelting with pyrite produces a matte containing essentially all of the silver, about 80 pct of the copper, and 37 pct of the iron; a coproduct is an iron-copper alloy containing all of the remaining iron and copper.
Extraction with metallic lead recovers essentially all of the copper from the molten iron-copper alloy with little or no loss of iron. If copper sulfides are present in the iron-copper metal, calcium carbide can extract the sulfur in a slag and liberate copper for subsequent dissolution in metallic lead. The decoppered, desulfured iron would be an acceptable foundry iron.
Treatment of the molten copper-iron alloy with sodium sulfate reduced copper and sulfur from 6.28 and 2.75 pct to 0.05 and 0.08 pct, respectively. For this range of refining, iron loss to the slag was about 25 pct.
