Table of Contents
The Bureau of Mines researched methods for recovering copper from cyclone and electrostatic-precipitator dusts of primary copper smelters as part of its program to maximize minerals and metals recovery from primary and secondary domestic resources.
Small-scale studies showed that use of 110 pct stoichiometric sulfur and 123 pct stoichiometric carbon, based on producing a 40-pct copper-iron matte, resulted in the recovery of 95 pct of the copper contained in the dust. Typical mattes contained less than 10 pct of the arsenic, 30 pct of the bismuth, and 45 pct of the antimony and tin contained in the dust. Matte was converted to blister copper by injecting up to 200 pct of the stoichiometric oxygen requirement over a period of 2 to 3 hours. Up to 76 pct of the copper contained in the dust was recovered as a crude blister copper containing about 2 pct arsenic, 0.5 pct antimony, and less than 0.1 pct bismuth and tin.
A matte-white metal product resulted from carbothermically reducing dust with coal, lime, and silica. Up to 95 pct of the copper was recovered in the combined product.
Direct carbothermic reduction and matte smelting were two pyrometallurgical techniques investigated by the Bureau of Mines on a small laboratory scale to recover copper and associated values from cyclone separator and electrostatic precipitator dusts. This research was part of the Bureau of Mines program to maximize the recovery of minerals and metals from primary and secondary domestic resources.
With the development of improved dust collecting systems and better pollution control equipment, a larger amount of dust is being returned for reprocessing. In commercial practice, when dust is returned to the reverberatory furnace, a part of this material is carried out of the furnace by gases and forms a recirculating load in the recovery system. Numerous studies have been made of methods for briquetting, agglomerating, sintering, and nodulizing dusts, and attempts have also been made to mix the dust with molten converter slag to form balls ranging from 1 to 6 inches in diameter which are returned to the blast furnace. Concern for the consequences of large recirculating dust loads in primary copper smelting practice prompted the present research, which is to take the dust aside and treat it in another furnace. This technique could reduce the recirculating dust load, and remove the associated impurities, resulting in a cleaner copper product.
The copper in cyclone and cottrell dust ranges widely (from 15 to 30 wt- pct) and accounts for 3 to 10 pct of the copper fed to the primary melting furnace. With improved dust recovery systems, only about 0.5 pct of the copper charged to the smelter is lost by dust entrainment in the smelter gas, and 1 to 2 pct is lost in the waste slags. The character of dust samples taken at different locations in a copper smelter has been described.
The behavior of minor impurities in matte smelting, converting, and anode production was detailed around the turn of the century. Later work has shown the need to control the impurities returning to the reverberatory furnace to insure the product quality and to meet stringent environmental standards. Other studies report that impurities such as antimony, arsenic, bismuth, and lead are difficult to remove from blister copper and if possible should be removed in preliminary ore treatment or matte smelting.
This work was directed toward recovering a recyclable copper product and determining the distribution of impurities in matte and blister copper when cyclone and cottrell dusts are treated in a separate electric arc furnace.
These small-scale studies demonstrated that smelter dust can be sulfidized to form copper matte with a limited impurity content. Most of the impurities were decreased to a low residual level by converting matte to blister copper. This product will require fire refining to remove the residual impurities prior to electrorefining.
Materials
The as-received dust was extremely fine cyclone and cottrell precipitator materials (95 pct minus 100 mesh) recovered from reverberatory operation of a commercial smelter. The dust samples contained 1 to 2 pct moisture and were dried overnight at 120° C prior to use. Bituminous coal from Utah served as the reductant and was crushed to minus ½ inch prior to use. Iron pyrite and chalcopyrite were used as the sulfidizing agents, and commercially available silica sand and pebble lime were used as fluxes. Analyses of the test materials are shown in table 1.
Characterization of Dust Samples
Examination of the as-received dust showed that there were two general fractions: one-third was glassy material, and two-thirds was opaque material. The glassy material, viewed in top and transmitted light, was composed of transparent oxide or silicate gangue material with particle sizes ranging from 0.1 to 5 µm in diameter. The opaque material was composed of copper prills, matte, and magnetite. The metallic prills ranged in size from 10 to 80 µm in diameter, and chemical analysis showed that they were close to the composition of white metal.
Microprobe studies showed that copper and iron occurred as mixed oxides and as sulfides of varying composition. Arsenic was disseminated throughout the sample and was usually apart from the copper. Small concentrations of Cu2S and ZnS (or possibly the sulfates) were also detected.
X-ray diffraction detected the presence of As2O3, Sb2O3, Fe2O3, Fe3O4, and trace amounts of PbSO4, and Cu2FeS·SnS.
Equipment and Procedures
Carbothermic reduction and matte-smelting studies were conducted in a size W 40-kva electric arc furnace. Single-phase current for the furnace was supplied by a 40-kva welding transformer, which was operated from a portable control panel located near the furnace (fig. 1). Manually operated rack-and-pinion-mounted

electrode clamps controlled the positioning of the 5-cm graphite electrodes. The steel furnace shell, approximately 43.2 cm OD by 43.2 cm high, was lined with a high-magnesia ram-cast mix, resulting in a working crucible 30.5 cm ID by 30.5 cm deep. The furnace shell was covered with a cast high-alumina roof supported by a water-cooled ring. A taphole was provided at hearth level to remove the molten products. The furnace offgases were evacuated by a 7,500- efm fan that exhausted into a positive-pressure cyclone and baghouse to collect entrained dust.
The dust, fluxes, and reductant were blended together and fed continuously to the furnace via a 10-cm-wide variable-speed belt. Feed rates were varied to minimize unfused charge buildup on the molten bath surface during the run. Feed rates were also correlated with the energy input to obtain smelting conditions to insure the best possible copper recovery. After the charge was melted, the energy input was decreased from 30-35 kw, and the bath was held for 20 min to allow the matte to settle out of the slag prior to tapping the molten products from the furnace.
Bath temperatures, as determined by an immersion thermocouple, ranged from 1,200° to 1,300° C during the feeding stage. During the holding period, the bath was held at about 1,200° C.
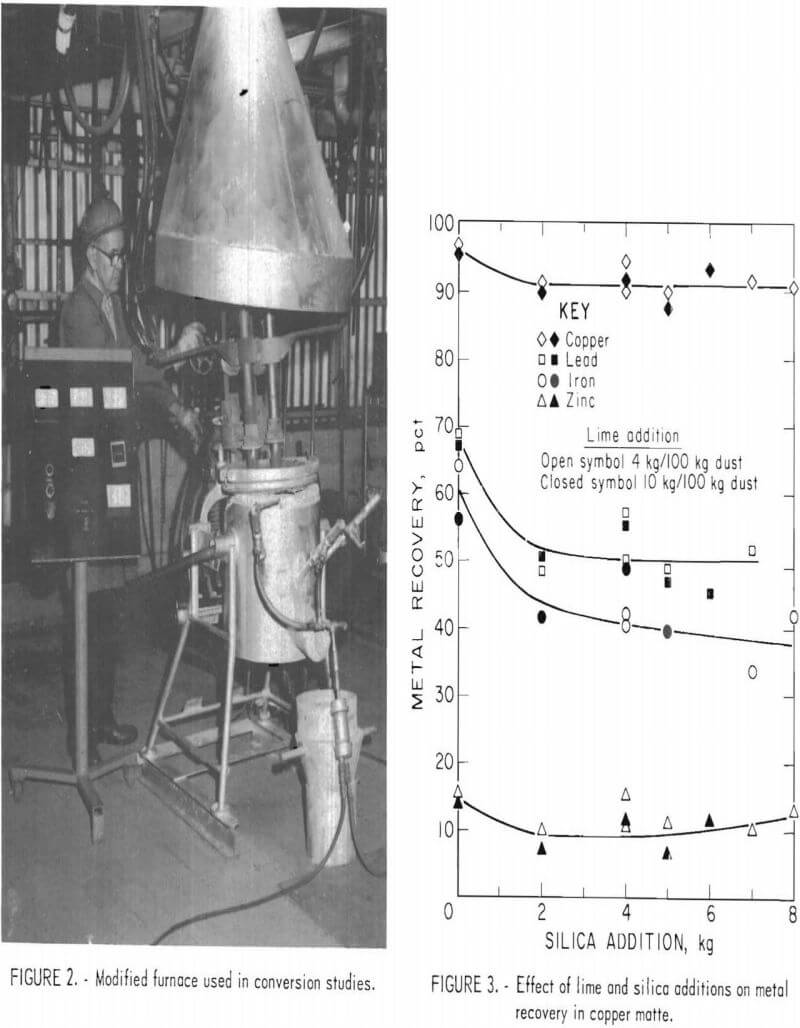
A modified furnace shell lined with a high-magnesia refractory and equipped with three tuyeres was used to convert molten copper matte to crude blister copper. One tuyere was located above the taphole, and the other two were located 45° to the right and left of the center tuyere (fig. 2), Each tuyere was inclined 45° from the vertical position and was equipped with a ball valve, which could be opened periodically to allow mechanical punching of the tuyere when it became plugged with slag and matte. An annular wedge section was removed from the top rear (top centerline to 10.2 cm down on the back side) of the furnace shell to allow the furnace to be tilted 23° to cover tuyere openings during converting operations. This modification of the shell permitted the roof and the graphite electrodes to remain in place throughout the entire converting operation. Therefore, electrical energy could be supplied, when necessary, to maintain the bath temperature in the range of 1,200° to 1,300° C.
For each conversion test, 20 to 30 kg of matte was melted and the molten bath was sampled. Air and oxygen were introduced to the furnace at 15 to 20 psi, and the weight of oxygen injected was determined by weighing the cylinders before and after each test. The air input was calculated from a totalizing flowmeter.
Silica sand was added at regular intervals during the test to flux the iron oxide and to maintain the weight ratio of FeO and SiO2 near 1.8. Slag was tapped from the furnace after the first hour. Thereafter, the slag tap-hole was kept open, and slag was allowed to overflow through the slag notch during the remainder of the run. Samples of matte were taken from the furnace at regular intervals and submitted for analysis.
Carbothermic Reduction Studies and Results
Laboratory-scale batch-smelting studies were made in the size W furnace to carbothermically reduce copper and iron from dust sample 1. Bituminous coal from Utah supplied 134 pct of the stoichiometric proportion of carbon to reduce Cu2O to Cu and Fe2O3 to FeO, assuming that nearly all copper and
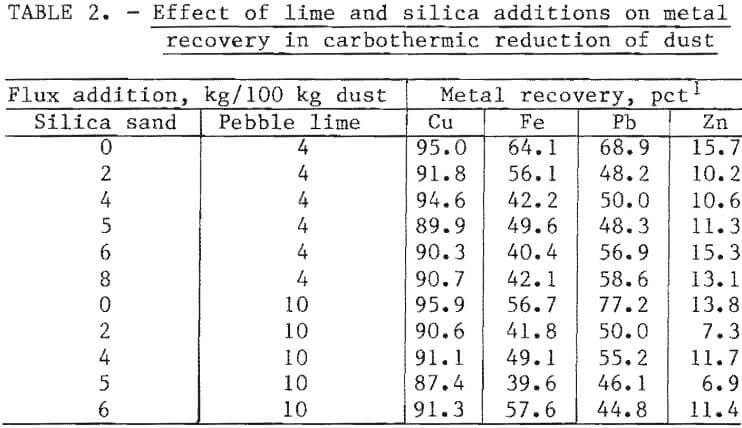
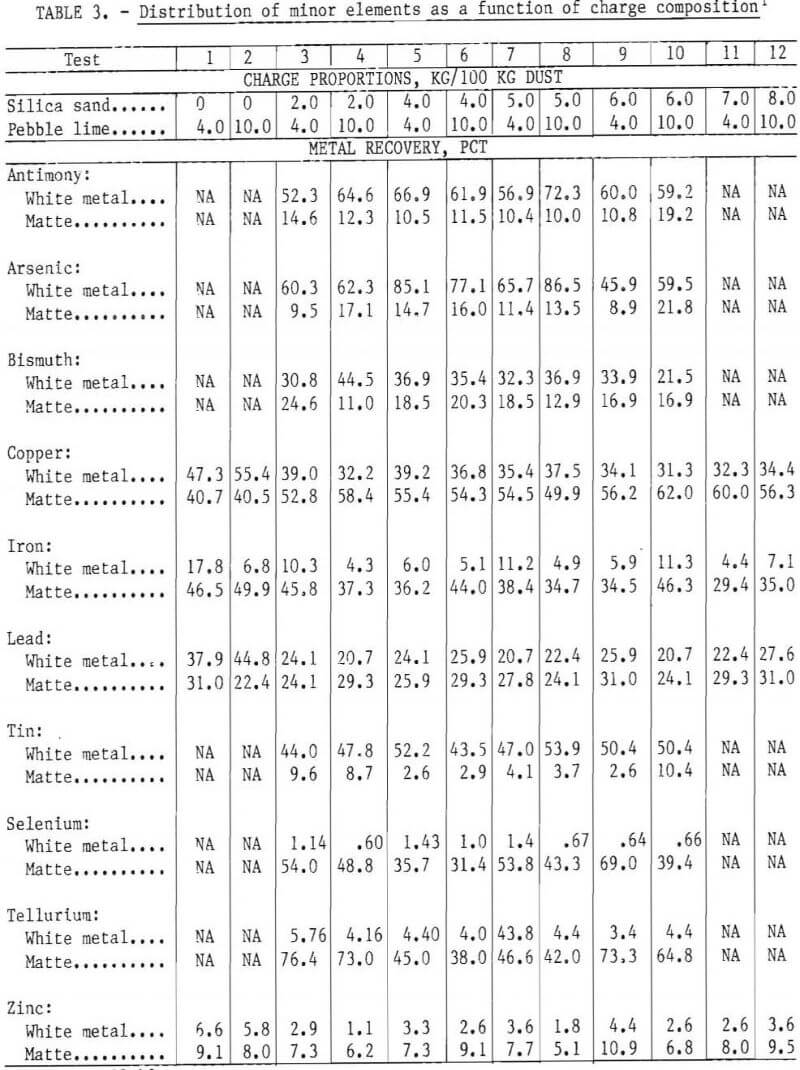
iron were present as these oxides. Two pebble lime additions (4.0 and 10.0 kg per 100 kg of dust) were used to keep the CaO-to-Al2O3 weight ratio greater than unity. Silica sand was added to the charge to adjust the weight ratio of FeO to SiO2 to between 0.9 and 1.5.
Up to 95, 69, and 64 pct of the copper, lead, and iron, respectively, were recovered from melting 100 kg of dust with 13.0 kg of coal and 4.0 kg of lime. The recovery of lead and iron decreased on smelting the charge with 4.0 kg of silica; copper and zinc recovery decreased only slightly on smelting with the minimum (4.0 kg) silica addition, as shown in table 2 and figure 3. Thereafter, recoveries showed little change with the use of greater silica additions.
About 50 pct of the copper, 40 to 44 pct of the iron, and 20 to 25 pct of the lead reported to the matte product; the remainder reported to the white metal or was lost to the dust that escaped the furnace. The distribution of arsenic, bismuth, selenium, tin, tellurium, and zinc between the matte and white metal is given in table 3.
Sulfidizing Studies and Results
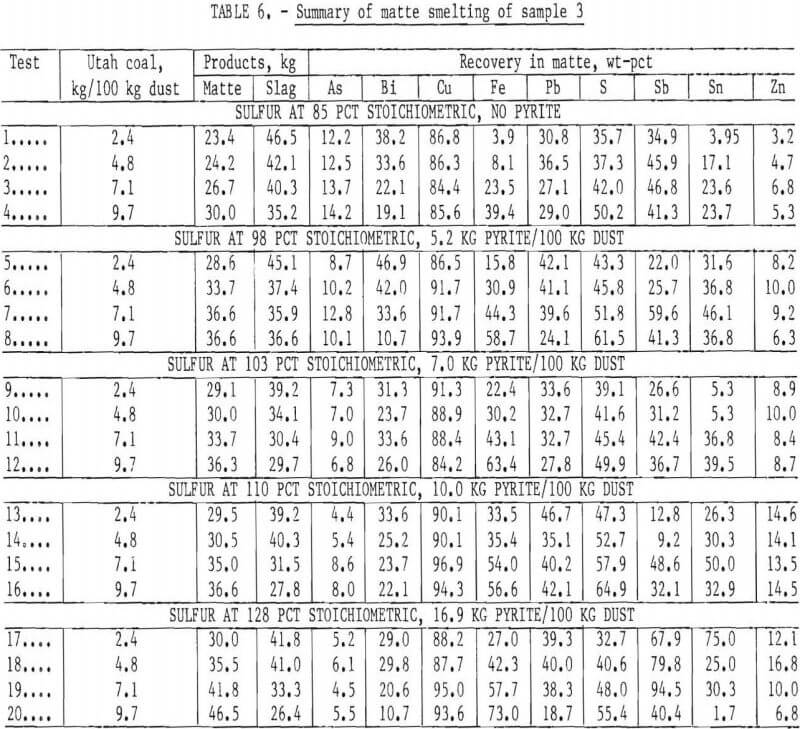
Sulfidizing studies were conducted on three dust samples from a commercial copper smelter to determine the feasibility of recovering copper by a matte-smelting technique in the electric arc furnace. Pyrite and chalcopyrite served as sulfidizing agents, and bituminous coal from Utah served as a reductant for these oxidized materials. The experimental procedure was similar to the procedure for carbothermic reduction studies.
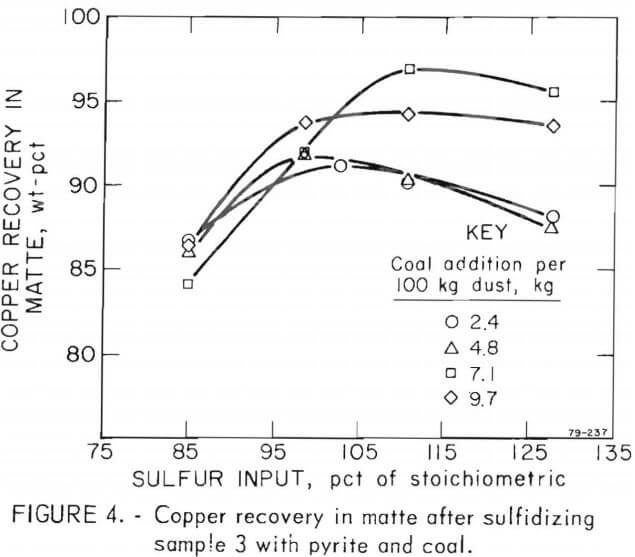
Copper recoveries ranged from about 71 to 85 pct after sulfidizing dust sample 1 with pyrite (table 4). After sulfidizing dust sample 2 with chalcopyrite to supply 98 to 142 pct of the stoichiometric proportion of sulfur for a 40-pct-copper matte (table 5), copper recoveries were from 77 to 87 pct. Stoichiometry was defined for purposes of computation as that quantity required to provide 28.2 wt-pct sulfur in a 40–pct-copper matte. Up to 95-pct copper recovery resulted from sulfidizing sample 3 with pyrite to supply 110 pct of the stoichiometric proportion of sulfur and 123 pct of the stoichiometric carbon, as bituminous coal, to reduce the ferric iron to the ferrous state (table 6).
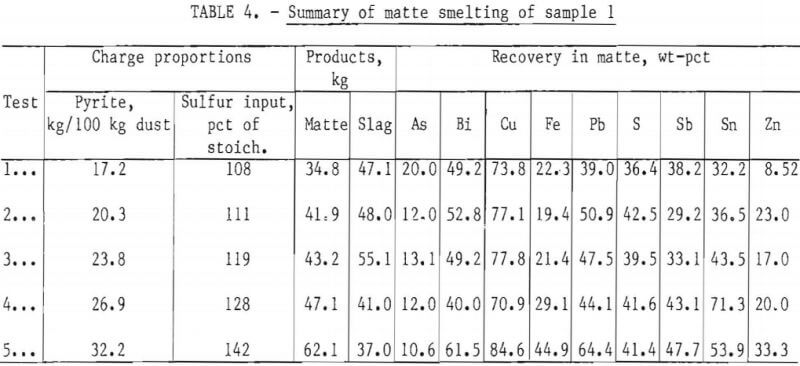
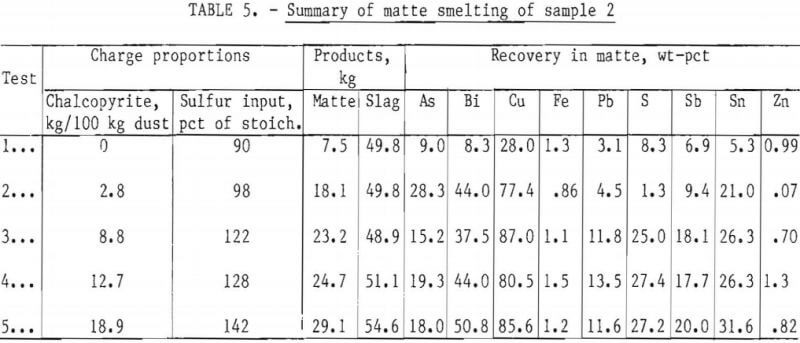
Copper recoveries improved as the sulfur in the charge increased from 85 to 110 pct of the stoichiometric proportion and the reductant was increased from 2.4 to 7.1 kg of coal per 100 kg of dust (fig.4). Increasing the pyrite input to increase the sulfur input from 110 to 128 pct of stoichiometric addition resulted in no significant increase in copper recovery. Increasing the coal addition from 7.1 to 9.7 kg per 100 kg dust favored the reduction of ferrous iron to the metallic state and consequently lowered the copper recovery.
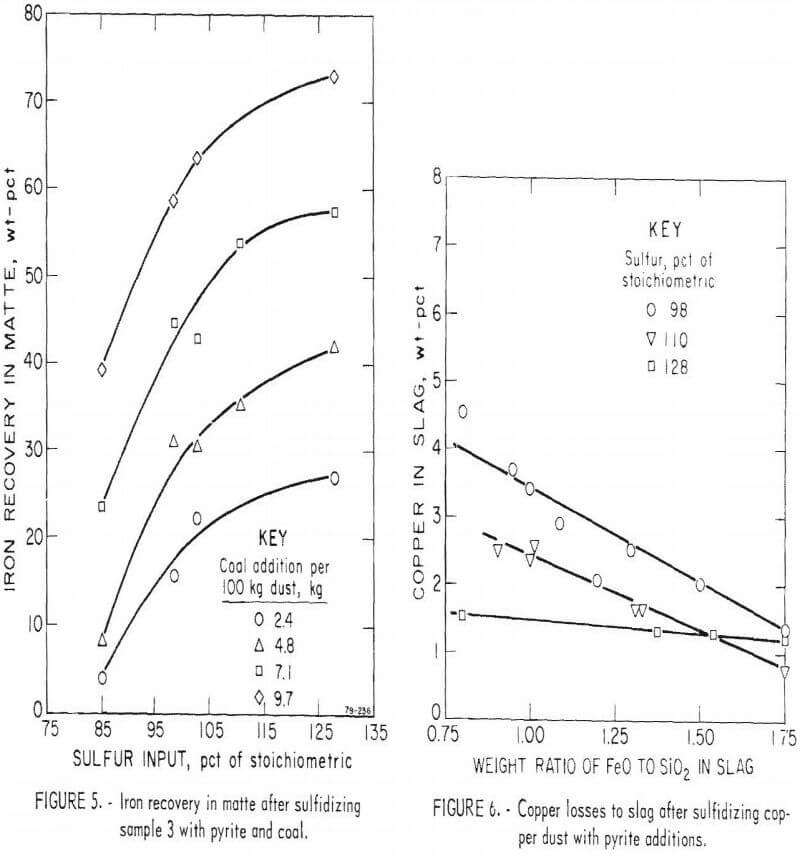
 Iron recoveries improved as the proportion of sulfur and coal in the charge increased (fig. 5). The iron content approached the theoretical requirement for a 40-pct-copper matte (31.8 pct) after sulfidizing the dust with 128 pct of the stoichiometric proportion of sulfur and the maximum level of reductant (9.7 kg coal per 100 kg dust).
Iron recoveries improved as the proportion of sulfur and coal in the charge increased (fig. 5). The iron content approached the theoretical requirement for a 40-pct-copper matte (31.8 pct) after sulfidizing the dust with 128 pct of the stoichiometric proportion of sulfur and the maximum level of reductant (9.7 kg coal per 100 kg dust).
Data in tables 4-6 show that less than 20 pct of the arsenic, less than 35 pct of the zinc, and less than 60 pct of the bismuth and lead reported to the matte. Arsenic recovery appeared to decrease as sulfur in the charge increased, but the recovery of bismuth, antimony, lead, zinc, and tin showed no change over the range of sulfur inputs studied.
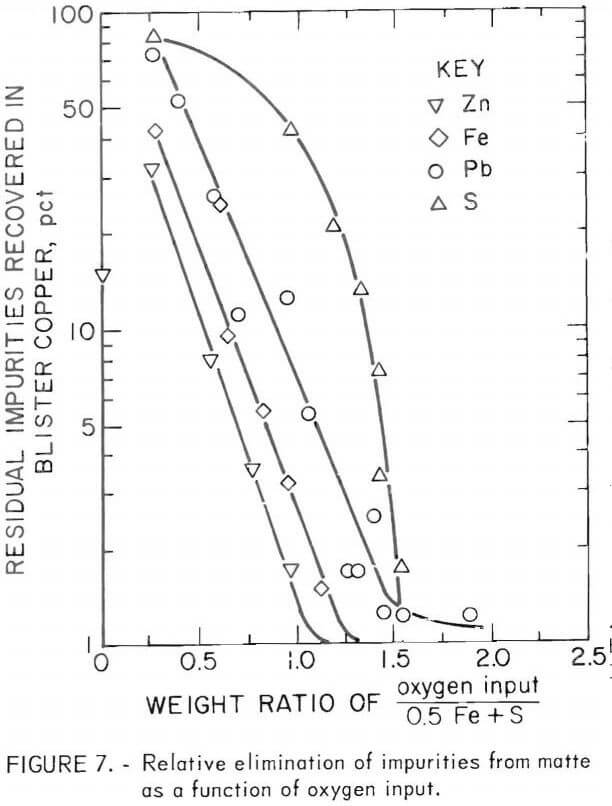
Copper losses to the slag decreased as the weight ratio of FeO to SiO2 in the slag increased from 0.75 to 1.75 when smelting with sulfur inputs ranging from 85 to 128 pct of the stoichiometric proportion required to form a 40-pct-copper matte (fig. 6). The improvement in copper recovery was greatest when smelting with substoichiometric sulfur input and the least significant when smelting with a 28-pct excess of sulfur.
Small concentrations (usually less than 0.10 pct) of lead, zinc, and arsenic (in decreasing order) were present in the slag along with other minor elements. The distribution of these elements to the slag was not studied in detail.
Converting Copper Matte to Blister Copper
A modified furnace shell equipped with three tuyeres was used to convert copper matte to blister copper. Both air and oxygen were introduced to the furnace at 15 to 20 psi. Silica sand was added at regular intervals during the test to flux the iron oxides and to maintain the FeO-to-SiO2 weight ratio near 1.8. Samples of matte and slag were taken at regular intervals and analyzed.
Results of Conversion Studies
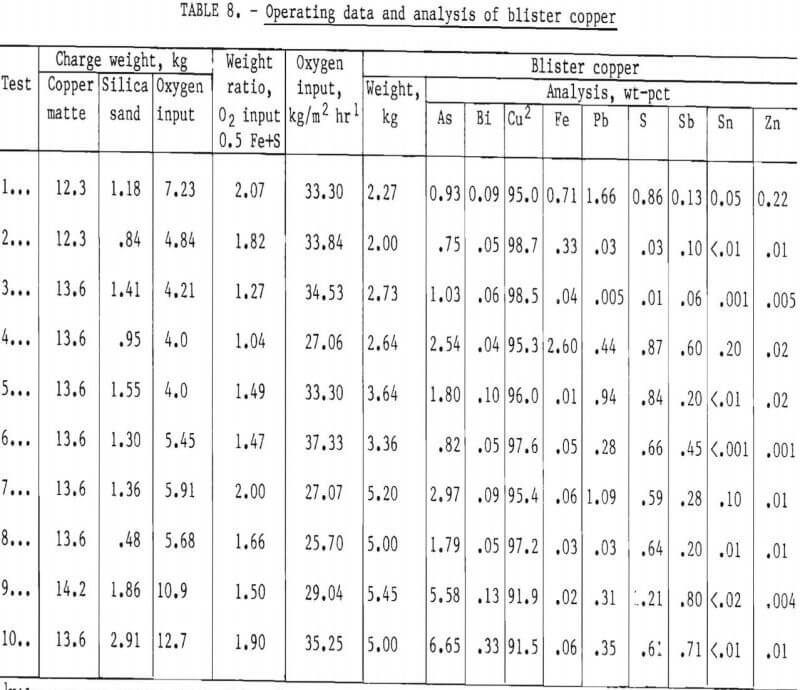
Copper matte was readily converted to blister copper by blowing the molten bath with air-oxygen mixtures. Up to 80 pct of the copper was recovered by injecting up to three times the theoretical oxygen requirement. Excess oxygen was recognized to be the result of inefficient use of the reagent in the shallow molten pool (about 2 inches deep), Arsenic, bismuth, antimony, and tin were eliminated during the conversion process to levels of 45.0, 90.4, 39.0, and 98.7 pct, respectively. Based on 100-pct copper recovery in the combined smelting and conversion processes, impurities eliminated were 99 pct of tin, up to 92 pct of arsenic and bismuth, and 78 pct of antimony (table 7).
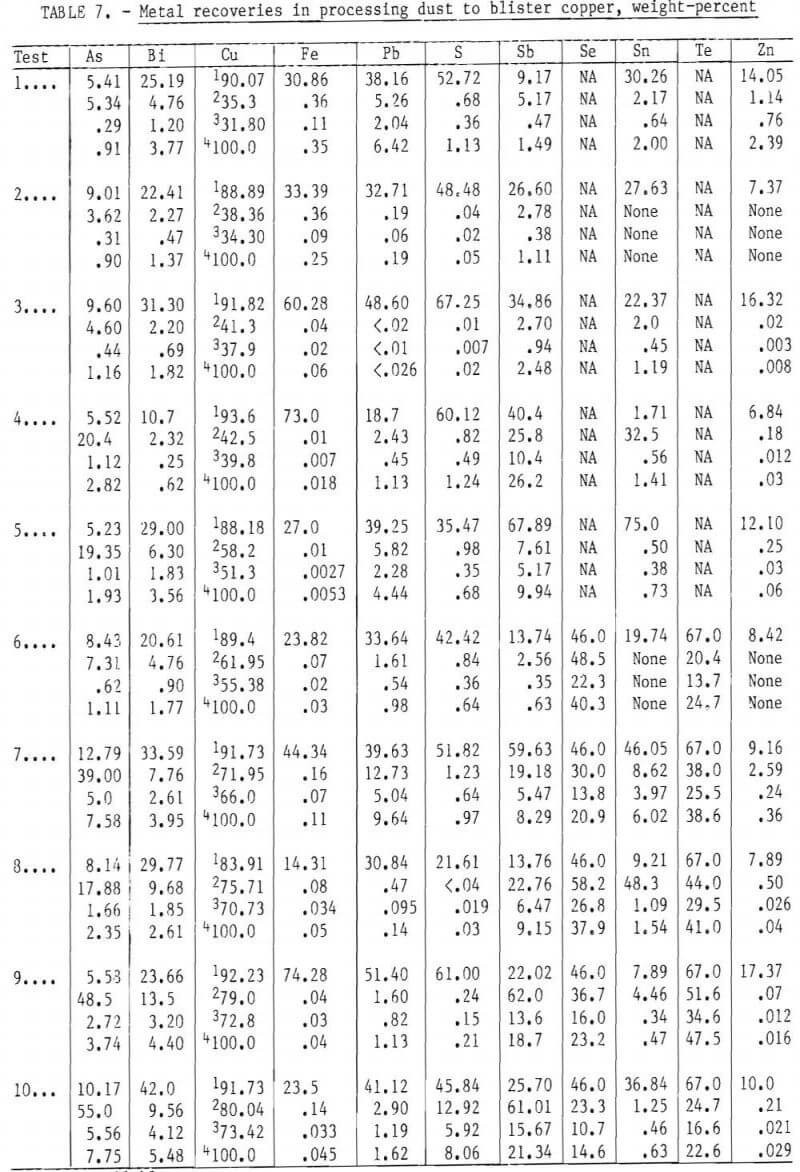
Data plotted in figure 7 show that zinc fumes off early in the operation. Iron was oxidized during the first hour of converting matte to blister copper, followed by lead, which was not completely removed in these tests. Once the iron and most of the lead were removed, sulfur oxidized from the white metal. Analytical data in table 8 show that arsenic was the principal impurity in blister copper, with smaller concentrations of antimony, lead, bismuth, and tin. Small amounts of both iron and sulfur were also present. The bulk of these constituents, except for bismuth, are removed by fire-refining treatment, which normally follows.
Discussion of Results
Oxygen was supplied to the conversion vessel at rates ranging from 30 to 35 kg/m² hr. This rate was insufficient to maintain the bath temperature in the range of 1,200° to 1,300° C. Therefore, the graphite electrodes remained in place throughout the run to maintain the bath temperature in the fluid range.
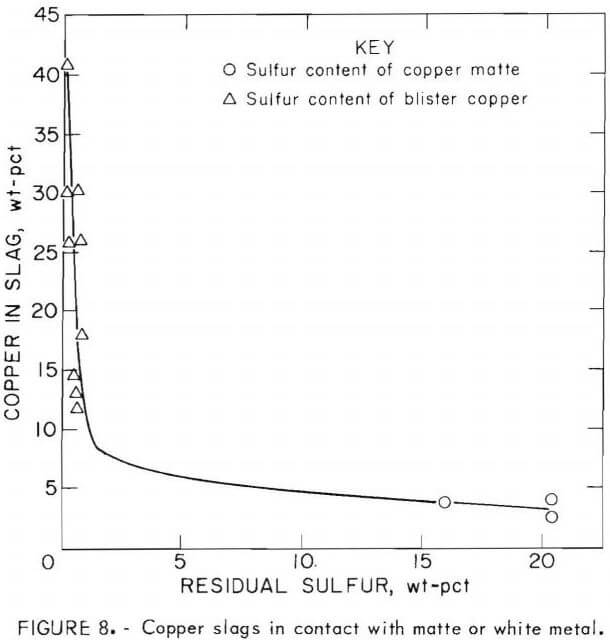
Improved copper recovery was mainly a function of slag composition and the degree of oxidation of iron in the slag, rather than total oxygen input. These studies and those of others show that prompt removal of the fayalite slag is essential if copper losses are to be minimized. In early work during this investigation (tests 1 to 5 in table 8), the slag remained in contact with the matte throughout the entire test, resulting in a highly oxidized slag containing from 15 to 19 pct copper. When the slag was removed at frequent intervals throughout the test, copper in slag was decreased to the range of 5 to 8 pct (fig. 8). Also, in blowing copper matte to blister copper, the copper oxide in the slag varied inversely with the sulfur in the matte. Copper losses were a minimum when the slag was in contact with high-sulfur matte, but increased significantly as sulfur in white metal decreased to less than about 5 pct (fig. 8).
Flue Dust Samples
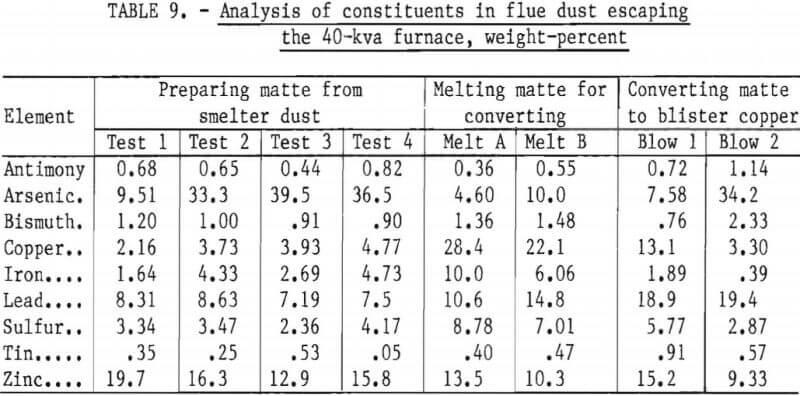
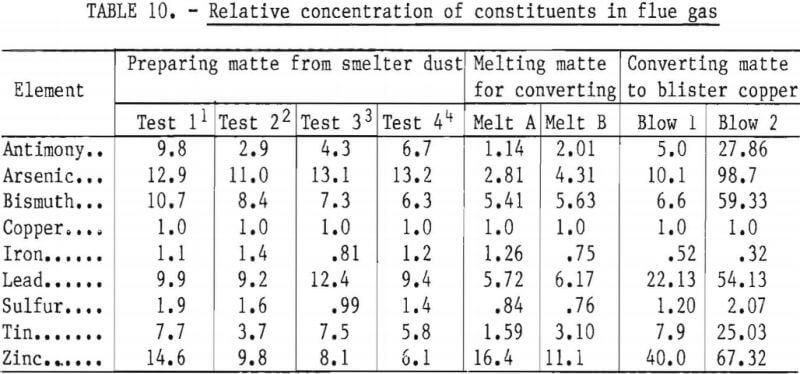
Data from carbothermic reduction, sulfidizing, and conversion studies show that up to 50 pct of the impurities escape the melting unit as dust or as vapors. Samples of dust and vapors escaping the 40-kva furnace were collected by a sampling device fitted with a filter paper and a suction system. The analyses of samples taken during the three furnace processing schemes are given in table 9. The relative concentration of impurities in the fume were calculated according to Keller. This evaluation was made by relating the analysis of flue dust and charge to a common basis by equating the copper to 100 and adjusting the concentration of the remaining elements proportionately. The relative elimination in smelting or in converting was found by dividing the data for flue dust by the data for the charge material. Data in table 10 show that when the species in the flue dust are compared to copper, arsenic is the most abundant, followed by lead, zinc, tin, antimony, bismuth, sulfur, and iron. With some minor variations, this ordering is the same for smelting with carbon, sulfidizing, and converting matte to blister copper.
Conclusions
Carbothermic reduction of a commercial smelter dust resulted in the recovery of 95 pct of copper, over 80 pct of arsenic and antimony, and over 50 pct of bismuth and tin in the combined metallic copper and matte production. Copper recovery improved as the weight ratio of FeO to SiO2 in the slag approached 1.7.
Sulfidizing dust sample 3 with pyrite and smelting with coal additions resulted in the recovery of 95 to 96 pct of the copper in a matte product containing about 40 pct copper. Copper recovery was greatest when sulfidizing with 140 pct of stoichiometric sulfur input and at least 7.1 kg coal per 100 kg of dust. Iron recoveries were improved with successive additions of reductant. The recoveries of tin, arsenic, bismuth, and antimony were 1.7, 5.5, 10.7, and 40.4 pct, respectively. From 50 to 60 pct of the selenium and tellurium in the dust reported to the matte.
The conversion studies showed that up to 80 pct of the copper in the matte was recovered by injecting 1.5 to 2.0 times the theoretical oxygen requirement. On the basis of 100-pct copper recovery in smelting and converting, the blister product contained less than 1 pct of the tin, 8 pct of the arsenic and bismuth, and 21 pct of the antimony in the dust.
These small-scale studies demonstrate that smelter dust can be sulfidized to form copper matte with a limited impurity content. The bulk of impurities were decreased to a low residual level by converting matte to blister copper. This product will require fire refining prior to electrorefining.
