Table of Contents
- MCIX Column Description and Operation
- Absorption Column
- Elution Columns
- Absorption Column Tests and Results
- Effect of Solution Flow Rate
- Effect of Aque0us-t0-Resin Flow Ratio
- Effect of Column Height
- Effect of Compartment Height
- Resin Inventory
- Resin Elution
- Pixed-Bed Column
- Pachuca Column
- Eluate Processing
- Sulfuric Acid Eluate
- Ammoniacal Eluate
- Process Economics
Significant amounts of cobalt, a strategic and critical metal, are present in some secondary sources such as spent copper leach solutions. The United States currently imports over 95 pct of its cobalt supply, much of it from Africa. Development of a process to recover cobalt from readily accessible spent copper leach solutions would help meet the Bureau’s goal of relieving the Nation’s dependence on foreign sources for strategic and critical metals. The total quantity of cobalt available in these solutions is not known, but cobalt recovery from one stream located at a major U.S. copper operation could produce about 1,000,000 lb Co annually. Additionally, five other domestic copper leach solutions containing significant cobalt values have been identified.
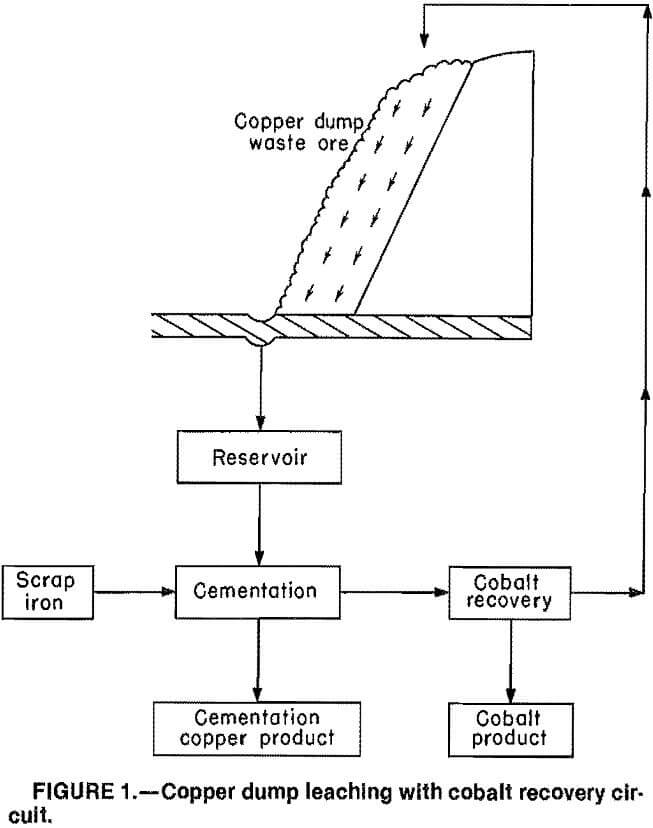
The copper leach solutions are produced by dump leaching of low-grade ores with dilute H2SO4. A schematic of a copper-leach circuit is shown in figure 1. Acid slowly percolates downward through the ore, leaching out metal values. The leach liquor is then collected in a reservoir and processed using cementation with scrap iron to remove most of the copper. At this point, a cobalt recovery circuit could be utilized to extract and recover cobalt from the spent copper leach solution; the barren liquor would be recycled to the leaching dump.
Recovery of cobalt from spent leach solutions has significant advantages over methods for recovering cobalt from primary sources. Since the cobalt is solubilized during the copper leaching operation, a separate dissolution step is avoided. Also, existing support facilities are available, and thus the initial capital investment for site development would be minimal.
Cost-effective technology for recovering cobalt from these low-grade solutions has not been available. Although significant amounts of cobalt may be recoverable, cobalt solution concentrations are only 10 to 30 ppm. Also, the solutions are complex and contain copper, nickel, iron, zinc, aluminum, and magnesium in addition to cobalt. Since economic considerations dictate that copper leach stream flows of several thousand gallons per minute must be processed, pH and temperature adjustments would not be practical. Likewise, the addition of reagents to the streams to enhance cobalt extraction would be costly and could affect affiliated leaching and copper recovery operations.
Processing techniques such as precipitation and solvent extraction have been developed for recovering cobalt from
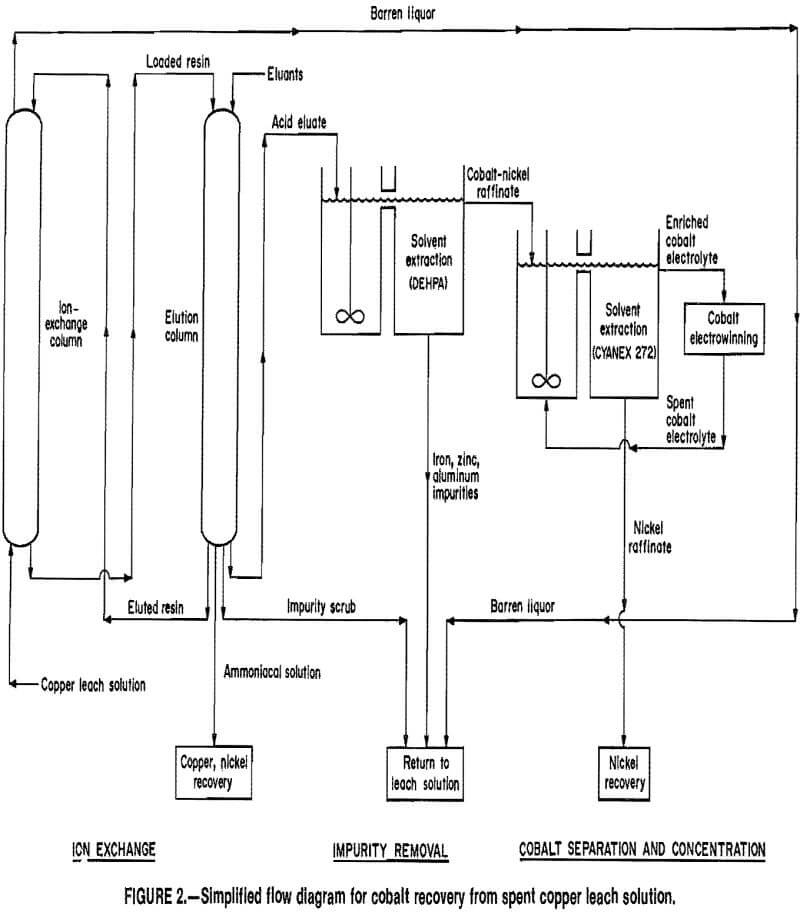
acidic sulfate solutions. However, these procedures are not amenable to dilute solutions because of large solvent losses, costly filtration, and poor cobalt selectivity. The Bureau has therefore investigated the use of ion exchange to extract cobalt from these solutions. The cobalt recovery process consists of four major unit operations: (1) ion exchange (loading and elution) to extract and concentrate the cobalt, (2) purification of the ion-exchange eluates using solvent extraction to remove coextracted impurities, (3) a second solvent extraction operation to separate the cobalt and nickel and concentrate the cobalt, and (4) cobalt electrowinning to produce a final product. A simplified flow diagram of the process is shown in figure 2.
The initial ion-exchange studies were conducted in 4-ft-high by 1-in-diam fixed-bed columns using Dow resin 4195.02. Over 90 pct of the cobalt was extracted from the spent copper solutions by this weakly basic chelating resin, but resin inventories were high and clarified solutions were required. Therefore, the ion-exchange investigation was directed toward alternative systems to reduce the resin inventory. Studies were conducted to determine the applicability of the Bureau-developed MCIX column. Previous Bureau work using this column to extract uranium from low-grade solutions had demonstrated its utility in reducing resin inventories when compared to fixed-bed systems. Also, turbid liquids can be processed in the MCIX column, and filtration of feed solutions is avoided.
MCIX Column Description and Operation
The Bureau’s ion-exchange circuit consists of an absorption column and an elution column. During the investigation, both a fixed-bed elution column and a Pachuca reactor were used for eluting loaded resin discharged from the absorption column.
Absorption Column
The MCIX absorption column was a 2-in-ID glass column containing either 1- or 2-ft-high compartment sections. Columns 18, 12, and 8 ft high containing various numbers of compartments were studied. The individual compartments were separated by orifice plates; each plate had a 0.5-in-diam opening which was equivalent to approximately 6 pct of the column cross-sectional area. The orifice plate at the bottom of each compartment in the pilot-scale unit was fabricated with a 60° slope that prevented dead areas from forming at the bottom of the compartments. The total column height included the thickness of the orifice plates and the compartment sections.
A simplified schematic of the column configuration is illustrated in figure 3. During operation of the column, resin was fluidized by maintaining a continuous up-flow of solution, except for brief intervals (3 to 5s) when the resin was withdrawn. During these resin discharges, the solution feed stream was momentarily interrupted, a valve at the bottom of the column was opened, and a one-compartment increment of loaded resin and solution was discharged from the column. This discharge transferred the fluidized resin in each compartment to the next lower compartment. Upon completion of resin withdrawal, the discharge valve closed, the solution valve reopened, and the solution upflow refluidized the resin throughout the column. Once the feed solution had passed up through the column, it flowed over a weir at the top of the column and was collected in a barren liquor tank. Careful control of the solution flow rate was necessary to ensure
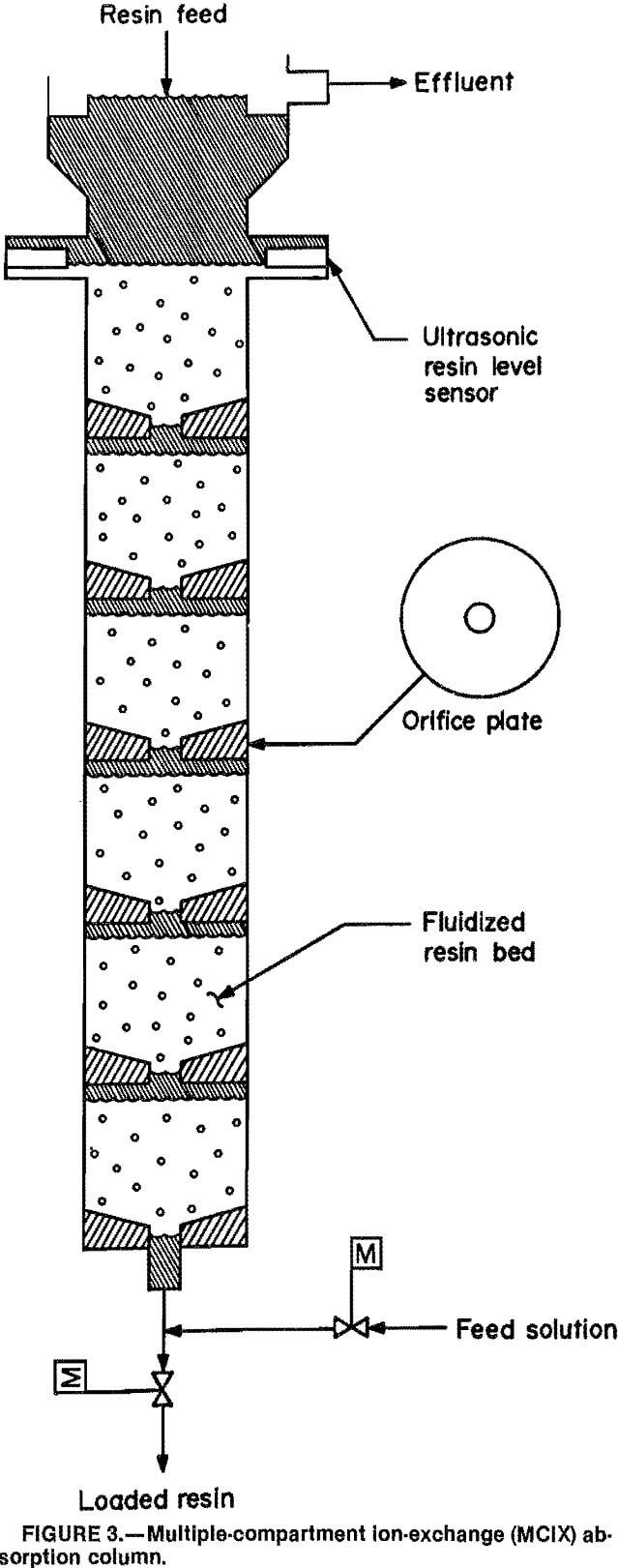
steady-state conditions in the column. Satisfactory control was accomplished using an automatic system consisting of a magnetic flowmeter, controller, and air-operated needle valve.
The time interval between withdrawals from the column was determined by the amount of resin to be discharged, which was a function of feed solution flow rate, feed solution concentration, and the desired resin loading. Column operation was most efficient when one entire compartment of resin and entrained solution was withdrawn each cycle. If the discharge was less than one full compartment volume, resin particle distribution in the discharge was not uniform. The upward flow of feed solution classified the resin within each individual compartment, and the smaller, lighter beads were not withdrawn during a partial resin discharge. These beads became saturated with cobalt and remained in the column, causing the cobalt absorption rate to decrease.
When the resin discharge cycle was completed, an ultrasonic sensor located at the top of the column detected the absence of resin in the top compartment. This sensor then activated a vibrator that fed eluted resin into the empty top compartment until the desired resin level was reached.
Elution Columns
During much of the investigation, resin discharged from the absorption column was transferred to a 5-ft-high by 3-in-ID fixed-bed column and eluted on a batch basis using techniques designed to ensure essentially complete metal elutlon. This procedure was necessary since a simultaneous study of both absorption and elution would introduce additional variables, and absorption results would be difficult to interpret. Resin discharged from the MCIX column was washed with water to remove entrained feed solution, stockpiled, and eluted in the fixed-bed column when several liters were available. The elution cycle consisted of a H2SO4 scrub, H2SO4 elutlon, and finally, NH4OH elution.
In the later stages of the investigation, loaded resin discharged from the absorption column was eluted in a 6-ft-high by 2.5-in-ID Pachuca column. The Pachuca assembly operated using an airlift mechanism that agitated the solution-resin mixture. A batch elution mode was utilized, but cycle times were shorter than those used in the fixed-bed column. As with the fixed-bed column procedure, the Pachuca elution cycle consisted of an acid scrub followed by H2SO4 and NH4OH eluants.
Absorption Column Tests and Results
The main objectives of the MCIX column investigation were to determine the minimum resin inventory and continuous operating conditions necessary to extract cobalt from a spent copper leach stream. These objectives were satisfied by studying four variables; (1) the solution flow rate, (2) the aqueous-to-resin (A:R) flow ratio, (3) the total column height, and (4) the individual compartment height. The tests were conducted by operating the column continuously for 50 to 60 h at a desired condition to ensure that steady-state operation was reached. The feed solution was pH 3.1 copper cementation plant effluent containing, in grams per liter, 0.03 Co, 0.03 Ni, 0.08 Cu, 1.48 Fe, 0.18 Zn, 3.13 Al, and 7.1 Mg. Both clear and turbid feed solutions were processed. The as-received solution was clear, but upon aging, ferric iron precipitated. A chelating ion-exchange resin from Dow Chemical designated 4195.02 was used throughout the test program. The resin mesh size was minus 20 plus 28.
Effect of Solution Flow Rate
The solution flow rate through the MCIX column significantly affected the operating characteristics of the column. A certain minimum flow rate was required to fluidize the resin, while fast flow rates were desired to minimize resin inventories and equipment costs. However, if the flow rate exceeded the resin terminal settling velocity, resin was entrained and carried out the top of the column.
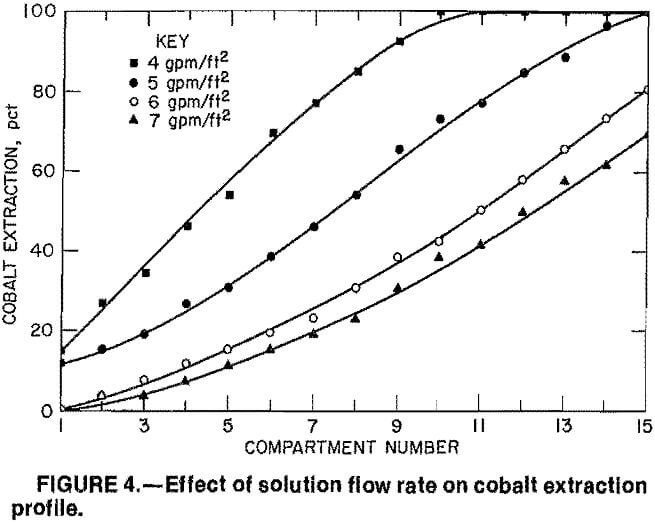
Using these limitations, the column was operated at flow rates of 4, 5, 6, and 7 gpm per square foot of column cross- sectional area. Each flow was held constant until steady-state conditions had been established; at this point, the solution in each compartment was sampled. A constant A:R flow ratio of 40:1 was maintained at each flow rate. Figure 4 illustrates the effects of the solution flow rate on the cobalt extraction. The compartment numbers shown in the figure correspond to the sequence of compartments in the MCIX column. Compartment 15 was located at the top of the column and compartment 1 at the bottom where the feed solution entered. As expected, cobalt extraction decreased significantly as the solution flow rate increased. At a flow rate of 4 gpm per square foot of cross-sectional area, essentially all of the cobalt was extracted from the feed by the 10th compartment; at 5 gpm/ft², all 15 compartments were needed to extract essentially all of the cobalt. The respective maximum cobalt extractions at 6 and 7 gpm/ft² were 81 and 69 pct. Cobalt loadings on the resin were 0.6 to 0.9 g per liter of wet settled resin (WSR). Nickel, copper, iron, and zinc impurity extractions followed the same trends as the cobalt extractions. Typical resin
loadings for coextracted impurities, in grams per liter of WSR, were 0.7 to 1.0 Ni, 2.0 to 3.0 Cu, 4.0 to 8.0 Fe, 4.0 to 5.2 Zn, 0.1 to 0.4 Al, and 0.1 to 0.3 Mg. No appreciable amounts of other impurities were detected.
Two factors strongly influenced the cobalt extraction as the flow rate increased. The first was the solution residence time, which was considerably shorter at the higher flow rates. For example, the solution residence time in the column was 29 min when the flow rate was 4 gpm/ft², but only 16 min at 7 gpm/ft². A second important factor influencing the cobalt extraction was the change in resin inventory. As the flow rate increased, the resin bed expanded and less resin was present in the column. At a flow rate of 4 gpm/ft2, the column contained 6.1 L WSR, but only 3.8 L WSR was. present at 7 gpm/ft². Thus, at the higher flow rates, not only was the solution residence time shorter, but the column contained considerably less resin.
Effect of Aque0us-t0-Resin Flow Ratio
A second variable that affected MCIX column operation was the A:R flow ratio, which influenced both the cobalt extraction efficiency and the cobalt loading on the resin. When high A:R ratios were used, cobalt loadings increased and less resin was required. However, as the A:R ratio increased, cobalt loadings approached the equilibrium loading limit of 1.2 g Co per liter of WSR. For example, the steady-state cobalt loading increased from 0.68 g per liter of WSR at an A:R of 40:1 to 0.94 g per liter of WSR at an A:R of 60:1. Nickel, copper, iron, and zinc loadings also increased proportionally at the higher A:R ratio, resulting in fewer available sites for cobalt absorption.
Figure 5 illustrates these effects when the A:R ratio was increased from 40:1 to 60:1 at a constant solution flow rate of 5.5 gpm/ft². More column length was required to achieve the same degree of cobalt extraction as the A:R ratio increased, and the extraction for 15 compartments decreased from 92 pct at an A:R of 40:1 to 77 pct at an A:R of 60:1. Similar results were obtained at flow
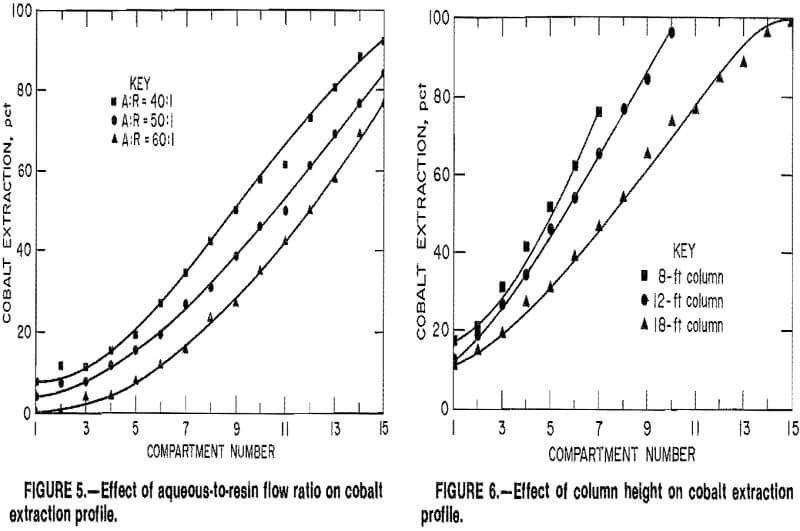
rates of 4 and 6 gpm/ft². For example, at 4 gpm/ft², the maximum cobalt extraction decreased from 100 pct at an A:R of 40:1 to 86 pct at an A:R of 60:1. Although a decrease in cobalt extraction occurred as the A:R ratio increased, cobalt recovery was less sensitive to variations in A:R ratio than to changes in solution flow rate.
Effect of Column Height
The MCIX column was also operated to determine the effect of column height on cobalt extraction. Tests were conducted in an 18-ft-high column containing 15 compartments, a 12-ft column containing 10 compartments, and an 8-ft column containing 7 compartments. These column heights were total heights and included the thickness of the orifice plates as well as the individual compartment heights. Solution flow rates of 4 and 5 gpm/ft² were tested at each column height, and a constant A: R flow ratio of 40:1 was maintained.
Cobalt extraction profiles for the three columns at a flow rate of 5 gpm/ft² are presented in figure 6. Cobalt extraction was more efficient per unit of height, and therefore per unit volume of resin, in the 12- and 8-ft columns. For example, only five compartments in the 8-ft column and six compartments in the 12-ft column were required to extract 50 pct of the cobalt, while eight compartments were needed in the 18-ft column. However, the total heights of the shorter columns were insufficient for complete extraction; a maximum of 96 pct of the cobalt was extracted in the 12-ft column, and only 76 pct in the 8-ft column. Similar trends were observed in the tests at 4 gpm/ft².
The increased efficiency of the shorter columns apparently resulted from variations in iron loadings on the resin. Ferric iron was coextracted from the copper leach solutions as the feed solution flowed countercurrently to the resin, and laboratory tests demonstrated that iron occupied sites available for cobalt extraction. In MCIX column tests, the iron extraction kinetics were slow relative to those of cobalt, nickel, copper, and zinc, and only 10 to 15 pct of the iron was extracted. Since iron was extracted at a slow but steady rate as the feed solution passed upward through the column, longer column lengths resulted in higher iron loadings. For example, resin discharged from the 18-ft column contained about 7 g Fe per liter of WSR, while resin discharged from the shorter columns contained only 3 to 4 g Fe/L. The decrease in the resin iron concentration then resulted in an increased cobalt extraction rate per unit of column height.
Effect of Compartment Height
The final MCIX column variable investigated was the effect of individual compartment height. In operation, the MCIX column is actually a series of agitated compartments or stages. Short compartments containing small amounts of resin are more efficient than longer compartments because of more favorable resin mixing and classification. However, compartment heights of 1 to 2 ft are preferred because of mechanical constraints such as resin handling.
MCIX column tests were conducted to compare cobalt extraction in a 12-ft column containing ten 1-ft-high compartment sections with a similar 12-ft column containing five 2-ft compartment sections. In each test the solution flow rate was 5 gpm/ft², and an A:R flow ratio of 40:1 was employed. Cobalt extraction profiles for each column configuration are presented in figure 7. Only a small difference in the extraction profiles was noted, although the 1-ft compartments were slightly more efficient in the bottom section of the column. The
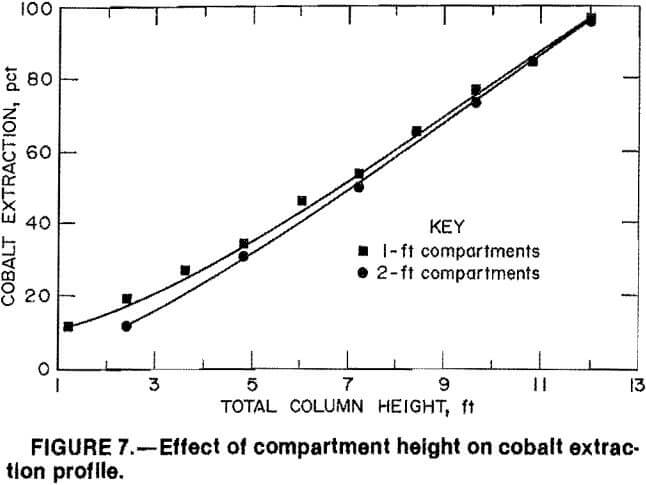
coextractions of iron and other impurities were similar for each compartment height. Also, mechanical considerations did not favor either compartment height, and thus no preference was determined in the pilot-scale unit.
Resin Inventory
Treating large volumes of feed solution containing only 26 to 30 ppm Co dictates that the resin inventory be kept to a minimum. Since the resin costs were a major factor in determining the process economics for the MCIX system, the resin inventories were determined for each of the test variables previously discussed. The resin inventory calculations were based on solution flow rates, resin flow rates, and cobalt extraction efficiency. These criteria were sufficient for defining the resin requirements for the absorption column, but the total resin inventory must also include that contained in the elution system. In a continuous system, loaded resin discharged from the absorption column would immediately be transferred to a continuous elution column, stripped of metal values, and returned to extract more cobalt. The resin inventory for elution would then be dependent on the resin flow rate and the time required for elution.
The resin inventory calculations included both absorption and elution requirements and were based on continuous processing of a 10,000-gpm spent copper leach stream containing 26 ppm Co. Although both batch and continuous fixed-bed elution of the loaded MCIX column resin were evaluated, continuous fixed-bed elution was assumed for these calculations since this elution mode would likely be used in a commercial facility. The minimum resin inventory, per unit of cobalt extracted, occurred when the 12-ft column was operated with a solution flow rate of 5 gpm/ft² and an A:R ratio of 40:1. Using these conditions, 16,500 ft³ of resin would be required to extract 96 pct of the cobalt from a 10,000-gpm stream. Generally, the lower resin inventories, per unit of cobalt extracted, were achieved with flow rates of 5 to 5.5 gpm/ft² and an A;R flow ratio of 40:1. Solution flow rates of 6 and 7 gpm/ft² and A:R ratios of 50:1 and 60:1 resulted in slightly higher resin inventories because cobalt extraction suffered significantly. Earlier test work on cobalt recovery utilized absorption and elution fixed-bed columns which required a much greater resin inventory. These columns would require 25,000 ft³ of resin to process a 10,000-gpm stream and achieve 96-pct Co extraction. Thus, use of the MCIX column reduced the resin inventory by 34 pct.
Resin Elution
Resin discharged from the MCIX absorption column contained nickel, copper, iron, zinc, aluminum, and magnesium, in addition to cobalt. Split elution techniques were therefore used to elute the resin: an H2SO4 scrub (pH 3.0) to remove a portion of the iron, zinc, aluminum, and magnesium impurities from the loaded resin; 30 or 40 g/L H2SO4 to strip the cobalt, remaining impurities, and part of the nickel; and finally, 3.5N or 4N NH4OH to remove copper and the remaining nickel from the resin.
Pixed-Bed Column
Several elution procedures were investigated in the fixed-bed column using various reagent concentrations and flow rates. The most effective procedure utilized two bed volumes of scrub solution, three bed volumes (two recycle and one fresh) of 30 g/L H2SO4, a bed volume of wash solution, and three bed volumes (two recycle and one fresh) of 4N NH4OH. The acid eluate contained essentially all of the cobalt, and one bed volume of this acid product eluate was obtained each elution cycle. A typical product eluate contained, in grams per liter, 0.9 Co, 0.4 Ni, 0.001 Cu, 4.5 Fe, 4.5 Zn, 0.1 Al, and 0.03 Mg. One bed volume of ammoniacal product was also obtained each elution cycle, and this eluate contained, in grams per liter, <0.001 Co, Fe, Al, or Mg, 3.4 Cu, 0.6 Ni, and 0.1 Zn. An eluant flow rate of 1 gpm/ft² and a total elution time of 180 min were used. Resin eluted using these conditions contained in grata per liter of WSR, 0.01 Co, 0.3 Ni, 0.3 Cu, 0.001 Fe, 0.01 Zn, 0.001 Al, and 0.001 Mg. The barren resin was returned to the absorption column, and these residual loadings did not have a detrimental effect on subsequent cobalt or nickel extraction. Samples of the barren resin were periodically analyzed for accumulations of other ions, but none were found.
Pachuca Column
Several elution variables were also investigated in a Pachuca column. These variables included the effects of the A:R ratio, elution time, eluant concentration, and number of contacts. One or two liters of loaded MCIX column resin were eluted in the Pachuca each cycle.
The most efficient elution procedure consisted of (1) a 5-min wash using pi 3.0 H2SO4 and an A:R ratio of 2:1, (2) two 5-min contacts using 40 g/L H2SO4 at an A:R of 2:1, (3) a 5-min water wash at an A:R of 2:1, and (4) two 5-min contacts using 3.5N NH4OH and an A:R ratio of 2:1. Two separate 5-min H2SO4 contacts were necessary since acid was neutralized during the elution, and insufficient acid was present after 5 min to effectively elute all the metal values from the resin. Acid eluates withdrawn from the column were therefore readjusted to a concentration of 40 g/L before use in the next cycle. Eluate products varied in cobalt and impurity concentration depending on the number of resin contacts; a typical eluate after 10 contacts contained, in grams per liter, 0.9 Co, 0.3 Ni, 0.01 Cu, 4.2 Fe, 5.6 Zn, 0.11 Al, and 0.03 Mg. The ammonlacal elution consisted of one contact with recycled NH4OH and one contact with fresh ammoniacal eluant. Ammoniacal product was therefore collected each elution cycle and contained 2.5 g/L Cu, 0.8 g/L Ni, and <0.1 g/L Co, Fe, Zn, Al, or Mg.
The elution time, including resin transfer and solution drainage using the Pachuca column, was 40 min, about one- fourth of that required in the fixed-bed column. This decrease in the elution time resulted in a resin inventory reduction of 74 pct in the elution circuit. The total resin inventory, including cobalt extraction in the MCIX column and elution in the Pachuca column, was reduced to about 12,000 ft³. However, as stated previously, elution in the Pachuca column was initiated in the later stages of the cobalt recovery investigation. Additional work is needed to fully define the effectiveness of the Pachuca column. Therefore, cost data presented in the process economics section are based on elution in a packed-bed column and reflect a total resin inventory of 16,500 ft³. Barren resin from the Pachuca elution circuit was returned to the MCIX absorption column and contained, in gram per liter of WSR, 0.02 Co, 0.3 Ni, 0.1 Cu, 0.01 Fe, 0.04 Zn, 0.004 Al, and 0.001 Mg. Transfer of slurried resin was accomplished using pressurized air.
Eluate Processing
The acid and ammoniacal column eluates contained considerable cobalt, nickel, zinc, and copper; further processing was necessary to recover these materials as marketable products. About 2.5 L of each product eluate (acid and ammoniacal) was produced for every 100 L of copper leach solution processed in the MCIX column. Since the volume of eluates was considerably less than that of the copper leach solution feed stream, precipitation and solvent extraction as well as ion exchange were evaluated as eluate processing techinques.
Sulfuric Acid Eluate
Selective precipitation of the cobalt was investigated from acidic column eluates containing 0.9 g/L Co, 0.4 g/L Ni, 0.001 g/L Cu, 4.5 g/L Fe, 4.5 g/L Zn, 0.1 g/L Al, and 0.03 g/L Mg. This procedure was unsuccessful because of the low cobalt concentration and presence of impurities. Several ion-exchange resins and solvent extraction reagents were then tested for selective removal of cobalt, but these techniques were also unsuccessful. Therefore, research was directed toward removing the impurities from the eluates; solvent extraction using di- 2-ethylhexyl phosphoric acid (DEHPA) was chosen. The DEHPA removed the iron, zinc, and aluminum impurities and was followed by solvent extraction with Cyanex 272 to selectively concentrate the cobalt. Finally, electrowinning was used to produce a metallic cobalt product.
Impurity removal by solvent extraction using DEHPA was accomplished in a 10-stage countercurrent circuit consisting of 4 loading stages, 2 acid stripping stages, and 4 sodium carbonate wash stages. The organic extractant was 15 vol pct DEHPA and 5 vol pct tributyl phosphate (TBP) in kerosene. The sodium form of DEHPA was used, as practiced by the Pyrites Co., Inc., to refine cobalt and nickel sulfate solutions. Use of the reagent in the sodium form eliminated the need for in-stage pH control since sodium rather than hydrogen ions were exchanged for the extracted species.
MCIX column eluates were adjusted to pH 2.1 with sodium hydroxide or sodium carbonate and contacted with DEHPA for 12 min in each of the four loading stages. An aqueous-to-organic (A:O) flow ratio of 0.6 produced a final raffinate of pH 7.0, and essentially all of the iron, zinc, aluminum, and copper were extracted. Cobalt and nickel reported to the final rafflnate, which contained, in gram per liter, 0.9 Co, 0.4 Ni, 0.01 Mg, and <0.001 Fe, Zn, Al, or Cu.
Loaded organic was stripped of zinc, aluminum, copper, and about 1 pct of the iron in the two acid stripping using 40 g/L H2SO4. The retention time was 14 min in each stage, and the A:O flow ratio was 1:1. Ten-volume-percent of the acid strip liquor containing 35.7 g/L Zn, 0.3 g/L Fe, and 0.01 g/L Al was continually bled off and replaced by makeup H2SO4. The bleed stream was then processed using a two-stage precipitation procedure to recover a ZnCO3-ZnO product. In the first step, essentially all of the iron and aluminum and a few percent of the zinc were precipitated by adding Na2CO3 until a pH of 5.0 was reached. The solution was filtered, and additional Na2CO3 was added to raise the pH to 7.5 and precipitate the zinc. Zinc in the acid bleed stream was recovered as an intermediate ZnCO3-ZnO product that contained 44 wt pct Zn, 0.2 wt pct Co, and <0,1 wt pct Ni, Fe, or Al.
Organic reagent exiting the acid stripping stages was contacted in four stages with a solution containing 80 g/L Na2CO3 and 40 g/L dextrose. The A:O ratio was 1:1, and the retention time was 15 min in each stage. Na2CO3 stripped the iron from the organic and converted the DEHPA to the sodium form, while dextrose chelated the iron and prevented it from precipitating. Stripped organic was returned to the extraction circuit, while the strip solution containing 3.7 g/L Fe and 0.3 g/L Zn was returned to the copper leaching circuit.
Separation of cobalt and nickel and further concentration of the cobalt were accomplished in a second solvent extraction system utilizing 17-vol-pct Cyanex 272 with 10-vol-pct nonylphenol in kerosene. Raffinate from the DEHPA circuit was fed directly into the Cyanex system, and interstage pH control maintained the pH of the aqueous phases in the loading stages between 5 and 6. Four loading and two stripping stages were required; the retention time was 17 min per stage, and the A:O flow ratio was 1:3. Essentially all of the cobalt was extracted by the Cyanex 272, while >99 pct of the nickel reported to the raffinate. Cobalt was stripped from the loaded organic with pH 2.0 depleted electrolyte, and the cobalt was recovered electrolytically. Nickel was precipitated from the raffinate with Na2CO3 and recovered as a high-purity NiCO3.
The enriched electrolyte produced in the Cyanex 272 circuit was fed into an electrowinning cell containing lead- antimony or lead-calcium anodes and stainless steel cathode blanks. The electrolyte contained 70 to 80 g/L Co, 0.05 g/L Ni, 0.1 g/L Mg, and <0.001 g/L Cu, Fe, Zn, or Al. Based on data from commercial electrowinning operations, this electrolyte was similar in composition to those of the industrial concerns. Laboratory tests determined that four criteria were necessary for electrowinning high-quality cobalt: (1) an electrolyte pH of 2.0 to 4.5, (2) an electrolyte temperature of 40° to 70° C, (3) current densities of 20 to 30 A/ft², and (4) electrolyte copper, iron, and zinc concentrations of not more than 0.001 g/L. When these conditions were satisfied, current efficiencies of 80 to 84 pct and cathodes assaying over 99 pct Co were obtained. These results compare favorably with those reported in literature. A bleed stream (about 1 pct of the electrolyte flow) was used to control trace impurity accumulations in the electrowinning circuit. The bleed stream contained 60 to 70 g/L Co, 0.04 g/L Ni, and 0.1 g/L Mg. A cobalt-nickel-magnesium byproduct was obtained from the bleed stream by precipitation with Na2CO3.
Ammoniacal Eluate
Elution of MCIX column resin with NH4OH produced eluates containing about 1.6 g/L Ni, 3.4 g/L Cu, and <0.1 g/L Co, Fe, Zn, Al, or Mg. Both evaporation of the eluates and solvent extraction separations were investigated for copper and nickel recovery. In the first procedure, ammonia was recovered from the eluates by distillation, and the remaining solution was evaporated to yield a residue. The dried residue contained 52 pct Cu, 24 pct Ni, and <0.1 pct Co, Fe, Zn, or Al.
The solvent extraction procedure used LIX 64N to extract the copper and nickel; this procedure produced an ammonlacal solution suitable for recycling to the elution circuit. Previous studies have indicated that LIX 64N is an effective copper-nickel extractant, and laboratory tests with ammoniacal eluates from the MCIX column elution circuit verified these results. Essentially all of the copper and nickel were extracted from the eluates using A:O ratios of 0.25 to 1.0, while copper was selectively extracted at A:O ratios of 1.5 to 2.0. Nickel was stripped from the loaded organic using 15 to 20 g/L H2SO4, while 150 g/L H2SO4 was used for stripping copper. However, initial cost estimates indicated that distillation of the ammonia and evaporation to yield a copper-nickel residue was more cost effective than solvent extraction.
Process Economics
A preliminary economic evaluation of the process to recover cobalt from spent copper leach solution was prepared by the Bureau’s Process Evaluation Group. The estimated capital cost for a plant processing 10,000 gpm of feed solution containing 26 ppm Co was $29.9 million based on first quarter 1986 costs. This plant would produce about 1,000,000 lb Co annually at an operating cost of $17.58/lb Co produced. Estimated byproduct credits for a copper-nickel residue, ZnCO3, NiC03, and cobalt-magnesium carbonate offset much of the operating cost and yield a net operating cost of $9.36/lb Co. (The current selling price of cobalt is about $10/lb; however, the price has been quite volatile in the last few years, ranging from about $6/lb to $40/lb.) Two areas were identified as major contributers to the process costs. About one-third of the capital costs were attributed to the initial resin inventory, and one-third of the total operating cost resulted from reagent requirements for removing iron from the ion-exchange column eluates.
A summary of the economic evaluation is presented in the appendix. A brief description of a 10,000-gpm commercial-scale plant is given, followed by a discussion of the capital and operating costs. Tables detailing these costs are also provided. This cost study was intended as a source of information to guide future research to improve this process, and significant process changes are still likely.
Summary and Conclusions
Continuous tests demonstrated that an MCIX column effectively extracted cobalt from spent copper leach solution containing only 26 ppm Co. A 12-ft-high, 10- compartment column containing Dow resin 4195.02 extracted 96 pct of the cobalt when using a solution flow rate of 5 gpm/ ft² and an A:R of 40:1.
In addition, MCIX column test results indicated the following:
- Cobalt extraction decreased significantly as solution flow rate increased.
- More column height was required to achieve the same degree of cobalt extraction as the A:R flow ratio increased.
- Decreasing the column height from 18 to 12 or 8 ft, while maintaining constant solution and resin flow rates, increased cobalt extraction per unit of height.
Solvent extraction procedures were utilized to remove impurities from the column eluates, separate the cobalt and nickel, and produce a cobalt electrolyte. Cathodes assaying over 99 pct cobalt were electrowon from this electrolyte.
A preliminary economic evaluation estimated the capital costs for a plant processing 10,000 gpm at $29.9 million. With credits for zinc, nickel, and copper byproducts, the net operating cost was estimated at $9.36/lb Co produced.
