Table of Contents
Our Solid Waste Program is directed toward three main areas of research:
- (1) Extraction of mineral, metal, and energy values from urban refuse;
- (2) recovery of mineral and metal values from wastes generated in the industrial utilization of raw materials and mineral-base products; and
- (3) upgrading and utilization of automotive scrap.
The objective of this phase was to develop technology for recovering and recycling metal values from obsolete electronic instruments. Such instruments are operational byproducts of military and space agencies, commercial communications, and industrial electronic operations of all kinds. Included are aircraft instruments, radar, communication, tracking and warning systems, industrial computers and controls, and selected components of manufactured consumer goods. Electronic components of this equipment often have copper pins, contacts, and connectors that are plated with silver, gold, or other metals to insure better contacts and corrosion resistance. Generally, these components are contained in aluminum cases. To recover the aluminum, the scrap is treated in an aluminum sweating furnace where most of the aluminum, precious metals, and part of the copper are melted and collected as ingots. Direct aluminum recycle of the sweated metal is not economical because it contains other metals that have values equal to or greater than that of the aluminum, and these metals would be lost in direct recycle to secondary aluminum smelters. For example, one lot of sweated scrap contained 70 percent aluminum, 20 percent copper, 12 ounces per ton gold, 140 ounces per ton silver, 1.5 percent lead, and 1.5 percent tin. The individual metal value per ton of sweated scrap would be aluminum, $280; copper, $200; gold, $420, lead and tin, $40; and silver, $200, for a total of $1,140. The scrap as secondary aluminum would have a probable maximum value of $200 per ton.
To extract aluminum from sweated electronic scrap, and, at the same time, concentrate the other metal values into a product that could be separated by commercial smelting and refining methods, two molten-salt electrorefining processes were investigated. One process utilized a three-layer refining cell where the molten scrap formed the bottom anode layer, the molten electrolyte the middle layer, and the refined aluminum the top cathode layer. Refining cells of this type have been used to prepare high-purity aluminum from primary aluminum. The refining of aluminum alloys scrap was investigated using this type of cell during World War II in Germany. The second process used a compartment cell where the molten scrap anode and the refined aluminum cathode were physically separated. An NaCl-KCl-AlCl3 electrolyte developed in the electrowinning of aluminum from AlCl3 was used. The theory of operation for both processes was based on the selective electro-oxidation of aluminum from the anodic molten electronic scrap into a molten salt electrolyte. A simultaneous reduction and recovery of aluminum from the electrolyte occurs at the cathode.
Recover Aluminum & Precious Metals from Electronic Scrap
Electronic Aluminum Scrap
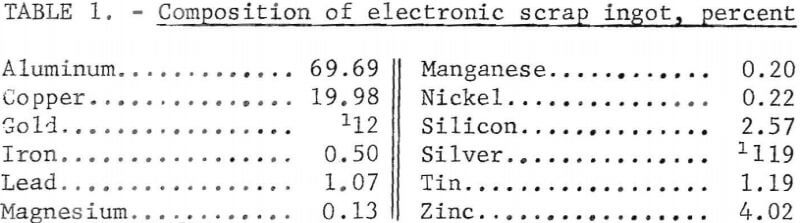
The electronic scrap used in these tests was from military aircraft scrap that had been sweated by a commercial smelter into ingots. In this report, the term “electronic scrap” will refer to these ingots. A 71-pound ingot that was representative of the electronic scrap was sectioned as shown in figure 1. Alternate slices of the ingot were selected for use in the two refining processes. This was done to minimize differences in the feed material resulting from possible segregation of metals in the ingot. The analysis of the ingot is shown in table 1.

Three-Layer Electrorefining Process
Apparatus
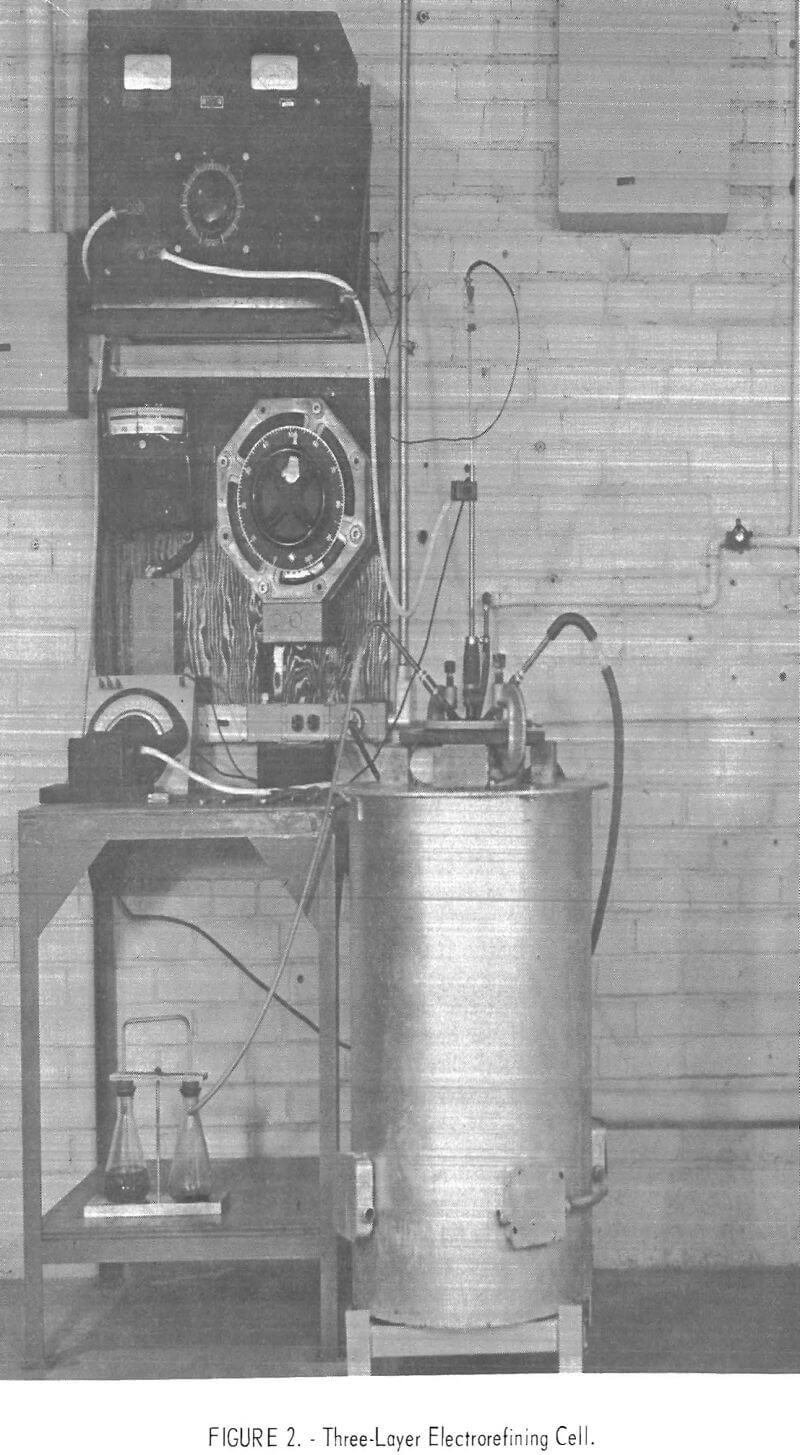
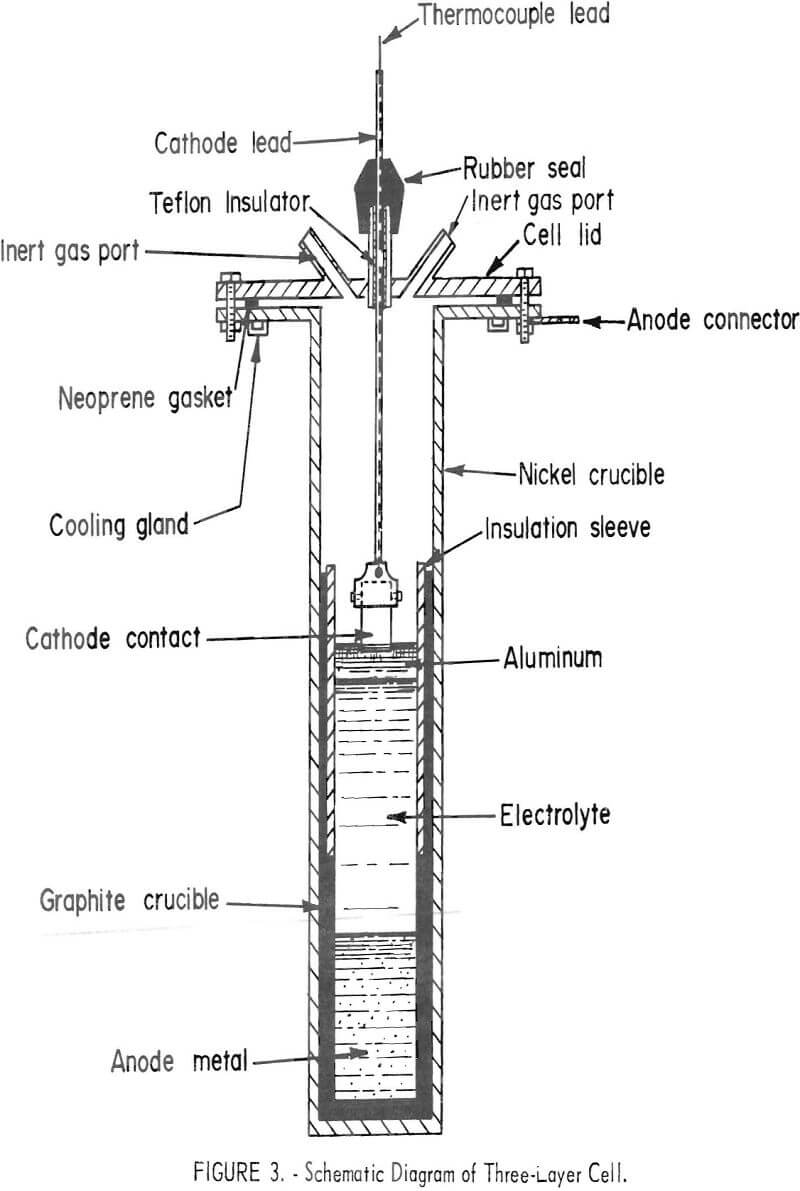
The three-layer electrolytic cell used is shown in figure 2; a schematic diagram of the cell is shown in figure 3, The crucible was 6 inches in diameter, 24 inches high, and constructed of nickel with a mild-steel flange and top. A high-density graphite liner was inserted inside the nickel crucible to contain the scrap, electrolyte, and refined aluminum. The gas ports were used to flush the air from the cell with helium to prevent oxidation of the graphite liner. The cell was heated by a resistance furnace. The anode lead to the electronic scrap was through the nickel crucible and graphite liner. An alumina sleeve was inserted in the graphite liner to insulate the cathode area from the anode. The cathode was a 1-inch-diameter graphite rod.
Operation
In the three-layer cell, the electronic scrap was the anode feed material. The three layers were separate because of a difference in density of the anode feed, the molten salt electrolyte, and the refined aluminum. At the operating temperature range of 750° to 850° C, the approximate density was 3.3 for the molten electronic scrap. 2.7 for the molten electrolyte, and 2.3 for the refined aluminum. The electrolyte contained 60 weight-percent BaCl2, 17 weight-percent NaF, and 23 weight-percent AlF3.
An initial charge of 10 pounds of scrap and 6.5 pounds of electrolyte was made to the cell. The cell temperature was raised to 800° C to melt the metal and electrolyte. The cathode was inserted 1 inch into the electrolyte and electrolysis was begun. A potential of 2.0 volts and a current range of 15 to 25 amperes were used for electrorefining. A series of 16 tests using the three-layer cell was made in which 2 to 3 pounds of refined aluminum were produced per test. Before each test was started, anode material was added to the anode layer of the cell in an amount equivalent to the aluminum removed at the cathode. At the conclusion of a test, the refined aluminum was ladled

from the cathode layer at the top of the cell. Ladling the aluminum from the cell is shown in figure 4.
At the end of the eighth test, 7-¾ pounds of anode residue was removed from the cell, and 6-2/3 pounds of new anode material was added. After the conclusion of test 12, no new anode material was added to the cell, and the following four tests were made to determine how completely the aluminum could be extracted from the feed material. After the 14th test, the anode residue, which had been removed at the end of the eighth test, was refed to the anode. Refining then continued through test 16 without any anode additions. Upon completion of the series, the anode residue was solidified and removed from the refining cell. Figure 5 shows the anode residue and the combined refined products from the first 12 tests.
Results
In the series of tests, 44.9 pounds of electronic scrap containing 31.3 pounds of aluminum was refined to produce 29.7 pounds of aluminum

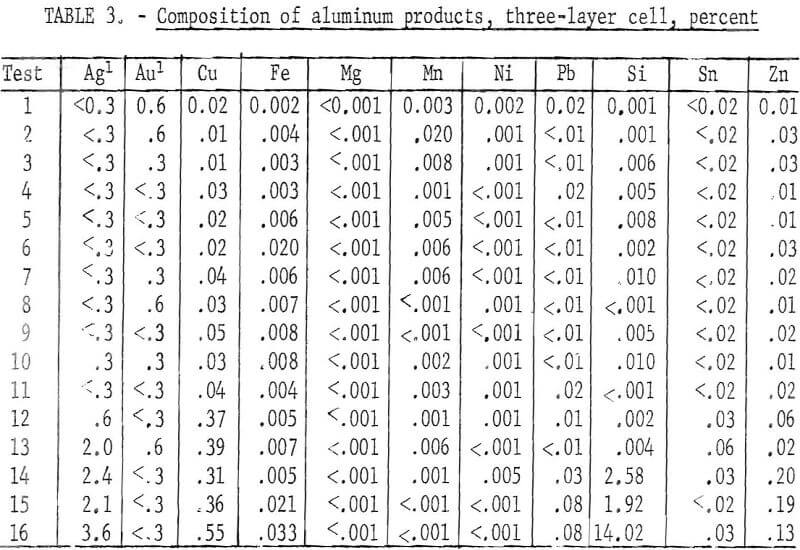
metal and 14.8 pounds of a concentrated copper product containing gold, silver, and other metals. The operating data are given in table 2. The quality of the refined aluminum in each test is shown in table 3. In the first 12 tests, which were conducted using the same parameters, refined aluminum of high purity was obtained. The major impurities in the aluminum were zinc and copper. The following four tests, which were made to determine the maximum amount of aluminum that could be extracted, showed an increase in impurities transferred to the refined products as the anode material was being depleted. The largest transfer was of silicon, along with smaller amounts of copper and zinc. The total recovery of aluminum from the series of tests was 94.1 percent. The average purity of the aluminum products of all tests was 99.4 percent.

The anode residue that was removed at the conclusion of the series had the composition shown in table 4. The other metals in the scrap were concentrated in the anode residue threefold after the removal of the aluminum with the exception of the magnesium. The magnesium in the scrap reacted with the aluminum fluoride in the electrolyte to form magnesium fluoride and aluminum, thus concentrating the magnesium in the electrolyte. At the conclusion of the series of tests, the only contaminant the electrolyte contained was 0.43 percent magnesium. Ninety-nine percent of the copper, 97 percent of the gold, and 94 percent of the silver were recovered in the anode residue.
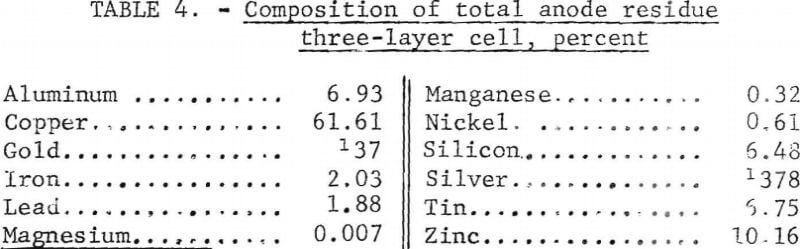
Examination of the anode residue showed a small layer of soft, dense metal on the bottom of the ingot. This metal was separated from the rest of the ingot. Analysis showed it contained 80 percent lead, 9 percent tin, 1.8 percent copper, 190 ounces per ton silver, and 5,5 ounces per ton gold. Thus, the recovery of most of the lead and part of the tin by liquation in a continuously operating cell seems possible.
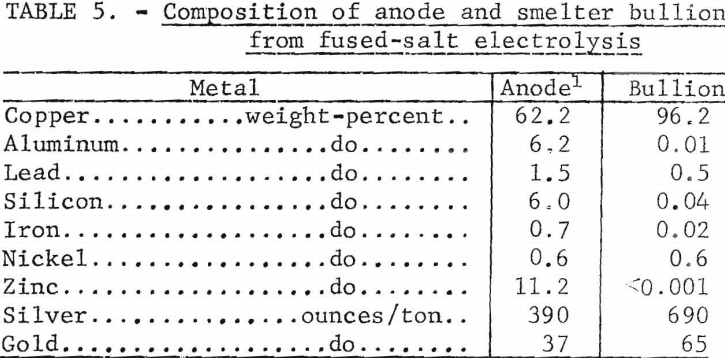
One hundred grams of anode residue was melted with a slag consisting of 10 grams of cryolite, 53.5 grams Na2CO3, and 10 grams SiO2 to remove aluminum zinc, and silicon. Air was blown through the melt for 30 minutes. Aluminum elimination was 95.7 percent. Copper, silver, and gold losses were 2.1, 1.6, and 2.0 percent, respectively. Table 5 shows the assay of the anode residue and resulting metal. The analyses in table 5 indicate that the metal recovered from the anode residue would be suitable for further treatment by aqueous acid electrolysis to separate precious metals and copper.
Compartment Electrorefining Process
Apparatus
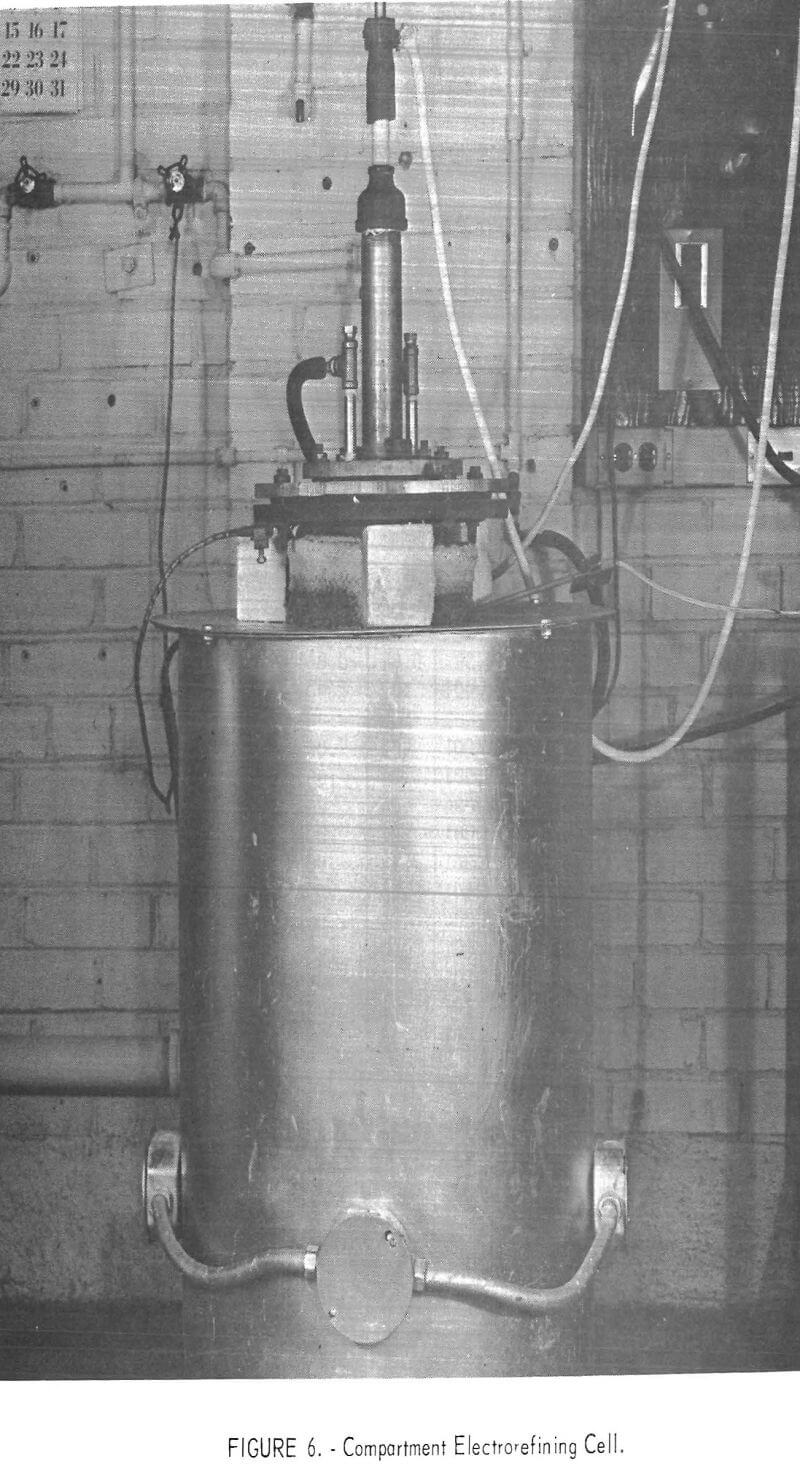
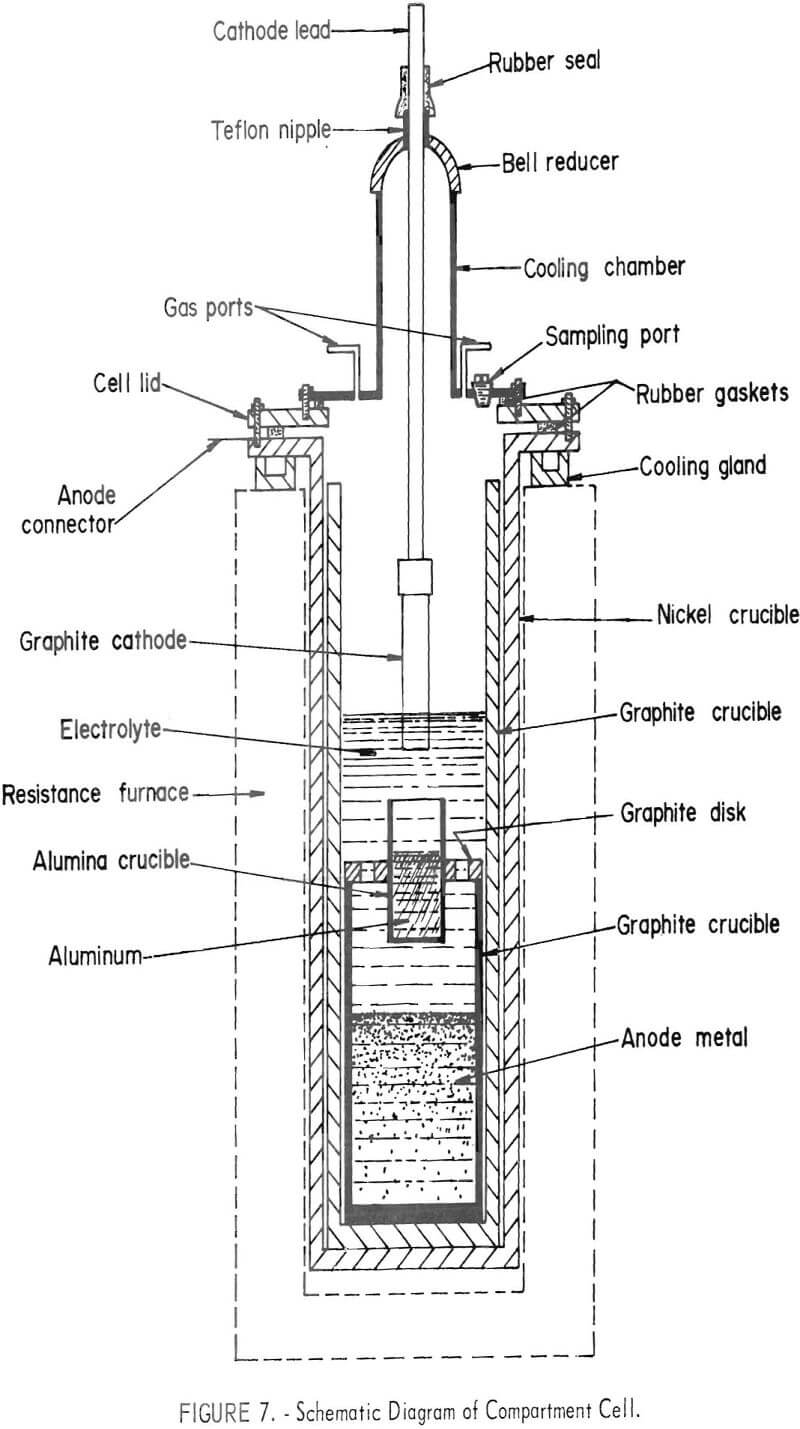
The compartment electrorefining process provided a separate compartment for the anode and cathode metals instead of the density separation used in the three-layer cell. The cell is shown in figure 6. A schematic diagram of the cell is shown in figure 7. The cell was similar in size and construction to the three-layer cell. The anode lead to the electronic scrap was through the nickel crucible and graphite liner. The cathode compartment was an alumina crucible suspended above the molten anode material and positioned so that the cathode metal would drip from the cathode and be caught in the crucible. Both graphite and titanium diboride were used as cathode materials.
Operation
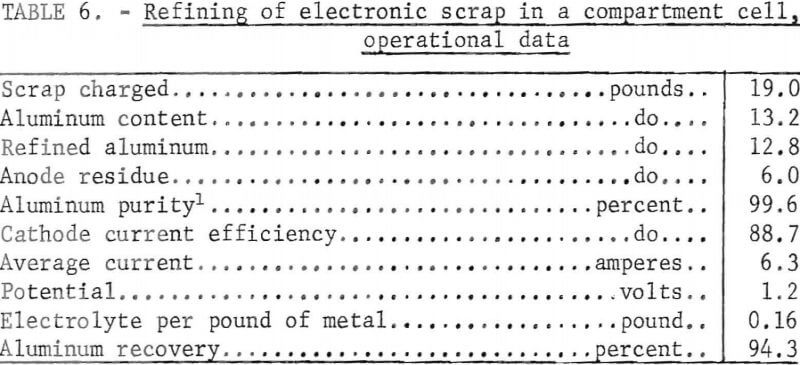
The cell was operated in the range of 750° to 800° C. The electrolyte consisted of an equimolar mixture of NaCl and KCl, with enough AlCl3 to provide a 1- to 2-weight-percent aluminum concentration in the electrolyte. An initial charge of 11.3 pounds of scrap and 8 pounds of electrolyte was placed in the cell. The 1-inch-diameter cathode was immersed 3 inches into the electrolyte. The operating potential was limited to 1.2 volts because of the decomposition potential of AlCl3. The current range was from 6 to 10 amperes. A series of 14 electrorefining tests was made, in which approximately 1 pound of refined aluminum was produced per test. During electrorefining, the aluminum was deposited on the cathode and dripped from the cathode to the bottom of the alumina crucible. After each test, the alumina crucible was lifted from the cell and the aluminum was poured into a mold. The removal operation is shown in figure 8. New scrap was then added to the cell in an amount equivalent to the refined aluminum removed. The alumina cathode compartment was then replaced and another test started. No additions of anode material were made after test 11. Tests 12 to 14 were made to extract the maximum amount of aluminum from the anode.
Results
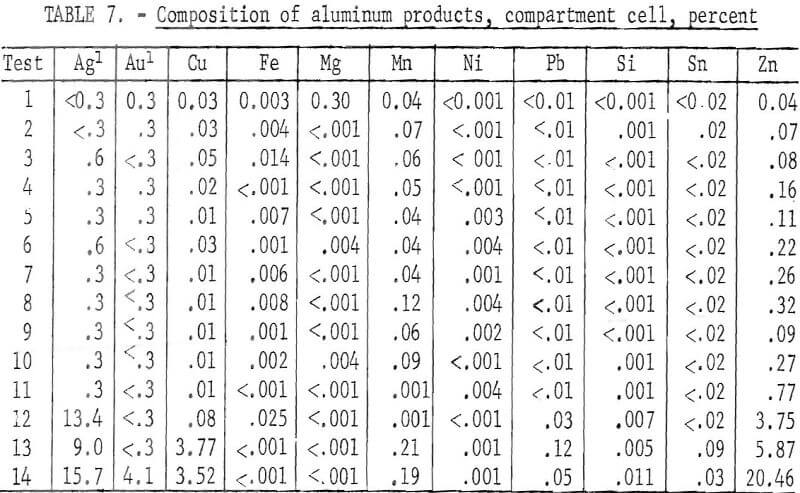
In the series of tests, 19 pounds of electronic scrap containing 13.2 pounds of aluminum was treated to produce 12.8 pounds of refined aluminum and 6 pounds of a concentrated copper, gold, and silver anode residue. The operating data for the series of tests are given in table 6. In the first 11 tests, which had the same operating parameters, the aluminum averaged 99.6-percent pure. The major impurities were zinc, manganese, and copper. The composition of the products is given in table 7. Tests 12 to 14 showed an increase in impurity transfer to the refined products as the aluminum content of the anode approached depletion. The largest transfer was of zinc and copper. The total recovery of aluminum was 94.3 percent.
The composition of the anode residue is given in table 8. The other metallic values in the scrap were concentrated in the anode residue by a factor of 3.1 after the aluminum was removed, The exception was magnesium, which reacted with the AlCl3 in the electrolyte to form magnesium chloride and aluminum. As in the three-layer cell, the only contaminant in the electrolyte was the magnesium. The magnesium in the electrolyte increased to 0.58 percent. This amount of magnesium in the electrolyte did not result in the contamination of the refined aluminum.

Discussion
Two electrorefining processes were shown to be technically feasible for the extraction of aluminum from sweated-aluminum electronic scrap. With either process, a high-quality aluminum metal of 99.6-percent purity or better was prepared. Operation on a continuous basis should recover over 95 percent of the aluminum. The quality of the aluminum prepared in the three-layer cell was better than that of the compartment cell. Under regular operating conditions in the three-layer cell, the only elements that transferred to the refined aluminum in appreciable quantities were copper and zinc. Likewise, in the compartment cell, the elements that transferred to the refined product were zinc, manganese, and copper.
On a comparable size basis, the rate of production for the three-layer cell was more than double that of the compartment cell. This was because of the limitation of potential for electrolysis in a chloride electrolyte as compared with a fluoride electrolyte used in the three-layer cell. Initial electrolyte costs are lower for the chloride electrolyte than for the fluoride-chloride electrolyte. The only contamination of the electrolytes was by magnesium. The useful life of an electrolyte would be unlimited except for dragout, if the scrap was heated in a molten salt containing either AlCl3 or AlF3 to remove magnesium before its addition to the refining cells. No evidence of magnesium contamination of the refined aluminum was found in the tests made with electrolyte magnesium concentrations of up to 0.58 percent.
The possibility of recovering the lead in the anode residue by liquidation was shown by the concentration of lead on the bottom of the anode residue.
The removal of the majority of the aluminum from the aluminum electronic scrap produces anode residue products that are amenable to commercial smelting and aqueous electrorefining methods for the recovery of the copper and precious metals.
Conclusions
Two molten-salt electrorefining processes are suitable for treating electronic aluminum scrap to recover aluminum as a high-purity product and to concentrate the majority of other metal values in a form that can be recovered by commercial smelting and electrorefining techniques.
Refining of the electronic scrap in the three-layer cell under regular operating conditions produced aluminum of 99.8-percent purity. Ninety-four percent of the aluminum present in the scrap was recovered at a cathode current efficiency of 90 percent. Other metallic values of the scrap were concentrated threefold in an anode residue that was suitable for treatment by commercial methods for recovering the copper and precious metals.
Refining of the electronic scrap in the compartment cell under regular operating conditions produced aluminum of 99.6-percent purity. Ninety-four percent recovery of the aluminum at a cathode current efficiency of 89 percent was achieved. Concentration of other metal values in the anode residue was similar to that in the three-layer cell.
