The activity of rare-earth compounds for catalysis of the hydrogen-oxygen reaction is of interest because of their possible use for electrocatalysis of the cathode reaction of a hydrogen-oxygen fuel cell. Although platinum metal has been widely used as a fuel cell catalyst, its high price and relatively limited supply prohibit its use in truly large-scale fuel cell applications. Nickel metal has also been used, but only with substantial sacrifice of fuel cell efficiency as a consequence of its larger oxygen overpotential. Development of an electrocatalyst having low oxygen overpotential as well as low cost could bring fuel cells closer to widespread use and help avert a possible energy shortage.
The development of efficient technology for separation of the rare-earth elements and production of pure rare-earth compounds, in which the Bureau of Mines was instrumental, has greatly increased the availability and reduced the cost of these elements.
This report presents the results of measurements of the catalytic activity of rare-earth oxides for the oxidation of hydrogen by oxygen at 150° to 250° C. The oxides of all the lanthanide elements (except the highly radioactive promethium) were investigated. Catalytic activities were determined on the basis of moles of H2 oxidized per square meter of catalyst surface per second. For the more active catalysts, activation energies were also determined.
Experimental Work
Catalyst Preparation
Catalyst samples were prepared from compounds of at least 99.5-percent purity (on a metal-element basis). Several methods of preparation were used, depending on availability of starting materials, ease of preparation, and physical properties of the products.
The hydroxides or hydrous oxides of La, Pr, Sm, Dy, Ho, Tm, and Yb were made by precipitation from an aqueous solution of their acetates with ammonium hydroxide. They were then filtered or centrifuged, dried, and finally calcined in flowing air at 600° C for 16 hours to produce microporous granules of the dry rare-earth oxides.
Cerium oxide was prepared by calcining the hydrated acetate in air at 800° C for 24 hours. The other elements were purchased as oxides. Some of these were further treated to convert them to a granular form. This was done by compacting the powder in a die under vacuum. Lutetium oxide was thus compacted at 1,250 psi, and neodymium oxide was compacted at 10,400 psi. The resulting compacted material was strong enough to withstand passage through screens for sizing while still retaining a satisfactory surface area.
Before use, each of the oxides was crushed and sieved to minus 20-plus 40-mesh size.
Activity Measurements
Activity measurements were made by passing a controlled mixture of hydrogen and oxygen (diluted with helium) through a fixed-bed microreactor of the Emmett-Kokes type and analyzing the effluent gas by gas chromatography. Figure 1 is a flow diagram of the apparatus.
The feed gas was delivered from two separate cylinders. One contained 1 percent O2 in helium; the other held 2 percent H2 in helium. The gas from each cylinder passed through adjustable metering valves before the two streams were mixed and fed to the reactor. Thus the O2-H2 ratio in the reactor feed gas could be adjusted to any desired value. Dilution with helium was employed to keep the hydrogen and oxygen concentrations safely below the lower limit of the explosive range. Reactor pressure was measured at the reactor inlet with a mercury manometer.
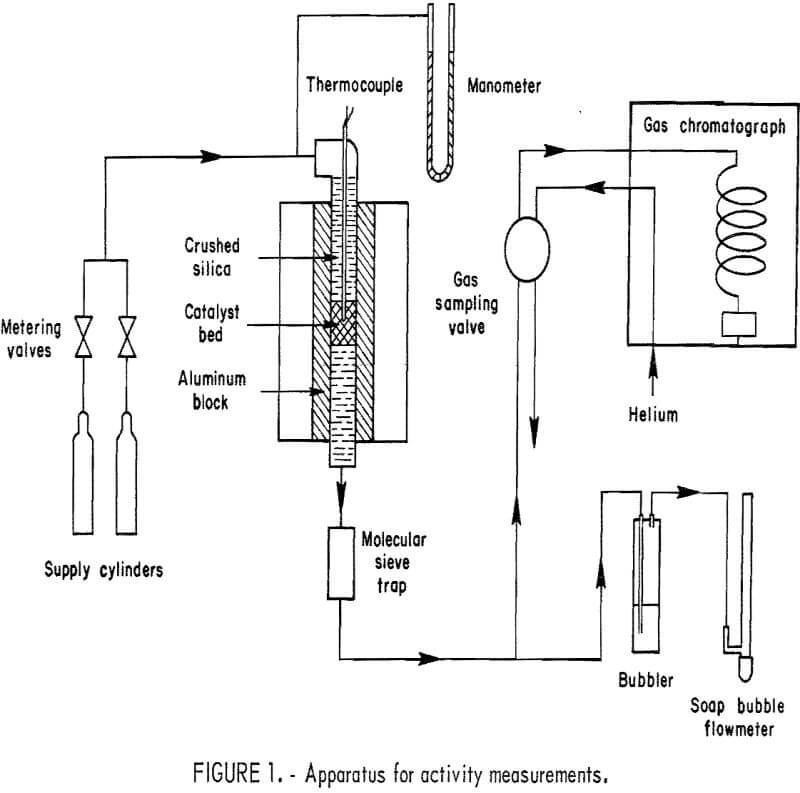
The reactor consisted of a ½- or 5/16-inch-OD stainless steel (type 316) tube 8 inches long, inserted in an aluminum block 1-¼ inches in diameter and 6 inches long. The block, heated by an electric tube furnace, gave a constant- temperature zone over nearly its full length. The top portion of the reactor was filled with fused silica particles sized to minus 20 plus 40 mesh, which acted as a preheater for the incoming gas mixture. Separated from the silica above and below by pads of glass wool, the catalyst bed occupied the central 0.5 to 1.5 cm of the reactor’s length. Temperature of the catalyst bed was measured with a type K thermocouple in a 0.0625-inch-diameter stainless steel sheath positioned near the center of the catalyst bed. On leaving the bottom of the reactor, the gases passed through a section of tubing containing 5A molecular sieve to remove water.
In a series of preliminary activity-screening tests, the product gas was passed from the drying tube directly through a gas-sampling valve on the chromatograph. For the measurements of specific activity, in which higher gas flow rates were necessary, the product gas was routed to a bubbler containing silicone fluid. A small portion of the gas flow was then drawn through the gas-sampling valve by gentle suction. In both cases, the gas finally passed through a soap-bubble flowmeter which was used to measure total flow rates.
In preliminary tests, a 1- to 2-gram mass of catalyst was placed in the ½-inch-diameter reactor tube. A flow of 1 percent O2 in helium at 20 cm³-min-¹ (NTP) was passed through the reactor while the reactor temperature was brought to 200° C. After all air had been purged from the reactor, the 02-helium flow was reduced to 10 cm³-min-¹, and a 10.2 to 10.5 cm³.min-¹ flow of 2 percent Hg in helium was added to the feed. The product gas was then analyzed at intervals during an on-stream period of ½ hour to 1-½ hours. The average reactor pressure was 690 torr with the 20 cm³-min-¹ total flow rate.
For the specific activity measurements, a 0.4-gram mass of catalyst granules was placed in the 5/16-inch-diameter reactor tube for the oxides with higher activity, and a 1- to 2-gram bed in the ½-inch reactor was used for the less active oxides. The procedure adopted for these tests was to load the catalyst into the reactor, purge with helium while raising the temperature to 160° C, and then start the oxygen and hydrogen flows. While maintaining a constant O2-H2 ratio with oxygen at 50 mole-percent in excess of stoichiometric, the feed rate was increased until the extent of reaction decreased to 10 to 15 percent. Then, holding the flow constant, the temperature was increased in increments and the extent of reaction was recorded at each temperature. Feed-gas flow rates up to 700 cm³·min-¹ were found necessary in some cases to bring the of reaction down to a measurable range. A lower limit of 50 cm³·min-¹ was imposed by the need to maintain precise control over the O2-H2 ratio in the feed-gas stream. The reactor pressure for these tests ranged from 670 to 737 torr.
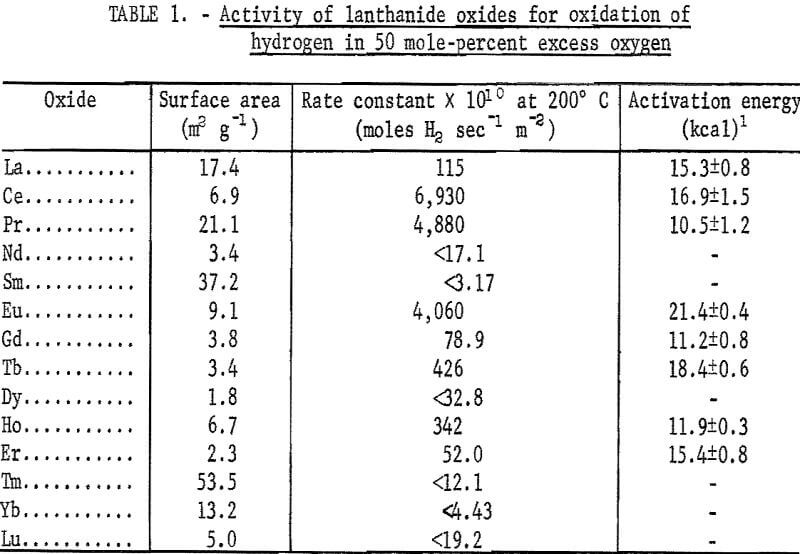
Specific surface areas of the catalyst materials were determined by a gravimetric nitrogen BET method. The results of these determinations are given in table 1.
Gas Chromatography
A Varian/Aerograph model 1420 gas chromatograph with a thermal conductivity detector was used for gas analysis. The chromatograph was fitted with a 1/8-inch by 12-foot stainless steel column packed with 30- to 60-mesh 13X molecular sieve and operated at 60° C with a helium carrier gas flow of 30 cm³·min-¹. A 2-ml gas sample loop was used for sample injection. Chromatogram peak areas were measured with a Varian/Aerograph model 477 electronic digital integrator.
Analysis of the product gas was complicated by the fact that only O2 could be determined accurately. Early attempts to measure the amount of water vapor produced by the reaction were abandoned because of problems with condensation and adsorption on tubing walls. For these reasons, a 5A molecular sieve drying trap was installed immediately downstream from the reactor. Hydrogen cannot be adequately determined with a thermal conductivity detector with helium as the carrier gas because of the similar thermal conductivities of the two gases.
To accurately determine the extent of reaction, krypton was used as an internal standard in the gas mixtures. It is inert, is readily separated from the O2 on the 13X mole sieve column, and gives a good detector response.
In preliminary tests, the O2-He supply cylinder contained an additional 1 percent Kr. The gas from this cylinder was mixed with the H2-He stream so that a slight (1 to 3 percent) excess of H2 was present in the final mixture entering the reactor. The excess H2 was necessary to insure that a sufficient amount of H2 was available for complete reaction of the O2. The extent of reaction was determined by comparison of the O2-Kr ratio in the product gas to that in the feed gas, the difference being proportional to the amount of O2 that had reacted.
In the specific activity tests, which were run under O2-rich conditions, only the H2-He gas stream contained Kr. To establish an O2-Kr response ratio indicative of the molar ratio of O2 to H2, the following standardization procedure was used: A slow flow (80 cm³·min-¹) of a mixture of O2-He and H2-Kr-He was passed over a 0.5 percent Pt on γ-alumina catalyst at 300° C to completely consume the reactant not in excess. The feed ratio of O2 to H2 was decreased until the O2 just disappeared from the product. The O2-Kr response ratio in the feed gas at that point was taken as the criterion for a feed mixture of exactly stoichiometric proportion.
Results and Discussion
The results of the preliminary tests are shown in figure 2. The graph shows percent hydrogen reacted as a function of time on stream. Immediately apparent is the wide variation of activity between different oxides. In spite of the similarity of their usual physical and chemical properties, the
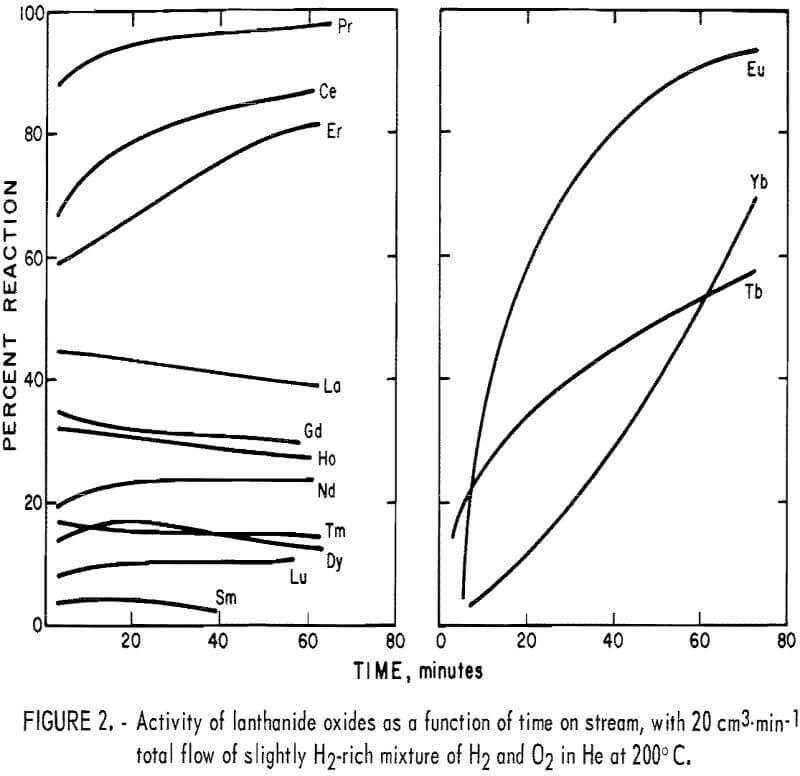
lanthanide oxides exhibit very different catalytic properties. This emphasizes the need for testing pure compounds of each of the individual lanthanides before drawing conclusions concerning their catalytic activity as a group.
A second observation resulting from the preliminary tests is that some of the oxides show a large increase in activity with time on stream. This may be due to a partial reduction of the oxide. All of the oxides were roasted in air during preparation, and the slightly reducing atmosphere of the reactor could remove some lattice oxygen from the surface of the catalyst.
The results of the specific activity tests are shown in table 1 and figures 3 and 4. The activation energies were determined by linear regression analysis of the logarithm of the measured reaction rate as a function of the reciprocal of reactor temperature in kelvins. These activation energies were used to calculate specific rate constants at 200° C via the Arrhenius rate equation. The rate constants are presented on a basis of unit surface area to allow for differences in specific surface area of the catalyst samples.
The maximum values of rate constants shown for some of the catalysts result from the 50 cm³·min-¹ minimum flow requirement of the reactor. That is, catalysts producing less than 10-percent reaction at 200° C with a 50 cm³·min-¹ flow are reported as having less than the rate constant corresponding to 10-percent reaction. As a consequence, it was not possible to determine the activation energy in those cases.
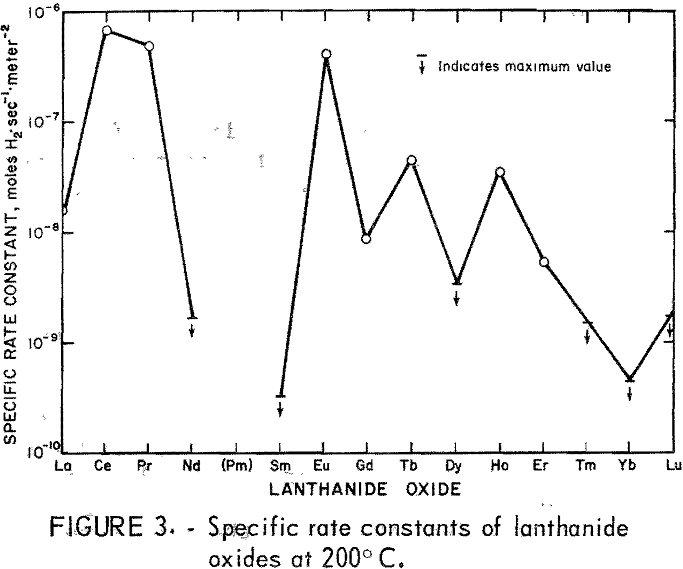
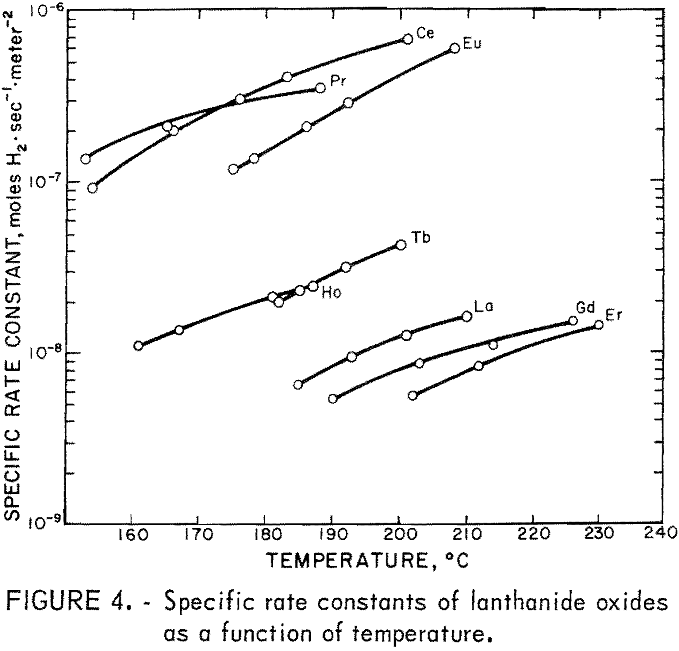
No attempt was made to modify the apparatus to cope with cases of very low activity, because catalysts having activity a thousand times smaller than that of the best catalysts are of no interest with regard to practical application.
The activities found for the catalysts tested thus far disagree with those found for presumably similar materials by Bakumenko, who reported an activity for cerium oxide under oxygen-rich conditions at 420° to 505° C of k = 1.7 to 0.8 x 10 -9 moles sec-¹ m-². While it may be not entirely correct to extrapolate our results to higher temperatures, an estimate of the activity at 450° C can be made by means of the Arrhenius equation. Based on our data, cerium oxide can be expected to have a specific rate constant of about 3.5 x 10 -4 moles sec-¹ m-² at 450° C. A discrepancy of five orders of magnitude between this and Bakumenko’s study is difficult to explain. A possible explanation is that the higher partial pressure of O2 in his experiments may have produced a catalyst surface substantially blocked by adsorbed O2. This suggests that a useful ancillary investigation might be the measurement of H2 and O2 chemisorption on our experimental rare earth catalysts.
Conclusions and Recommendations
The goal of this project is to develop a rare-earth catalyst suitable for use in the cathode of a hydrogen-oxygen fuel cell. The first step was to study the catalytic activity of rare-earth oxides for the oxidation of hydrogen in the gas phase. Activity was found to be greatly different for the various oxides. Activation energy for the reaction also varied. Some of the oxides exhibited significant levels of activity and these should be investigated thoroughly.
In addition to pure rare-earth oxides, other catalyst preparations should be studied, including alumina-supported oxides and mixed rare, earth-transition metal oxides.
Aside from the gas phase activity measurements, physical properties of the catalyst materials must be evaluated. Electrical and surface properties and strength of the materials will be important factors in the development of a fuel cell catalyst. Finally, electrodes containing the most promising rare-earth catalysts should be tested for their effectiveness in the electroreduction of oxygen under fuel cell conditions.
