Table of Contents
In the course of work completed, the problem so far met with has been the determination of the various elements (or compounds) present in a substance. In an ore, for instance, there may be present silica, iron, copper, arsenic, and sulphur, and it is the province of qualitative analysis to determine the presence or absence of these and other elements. It is obvious, however, that there still remains an unknown factor, namely, the quantity of each element present in any given substance. Having found that the ore contains the substances mentioned, the student is now asked how much silica, iron, copper, arsenic, and sulphur by weight are present in one gram or other convenient weight of the ore.
It may be said, then, that the province of quantitative analysis lies in the determination of the quantities— either by weight or by volume—of the various elements present in a substance. This substance may be a solid, liquid, or a gas.
Such an analysis may be either proximate or ultimate. For example, if one gram of salt be mixed with one gram of iron pyrites and the mixture be analysed, we may:
- Extract the salt with water and leave the iron pyrites as a residue. By filtering and evaporating the solution to dryness, the salt may be estimated; and by drying and weighing, the iron pyrites may be estimated.
- The formula of salt is NaCl, and of iron pyrites FeS2. By methods given further on, we may estimate the Na, Cl, Fe and S present by four separate determinations.
In the first case, where compounds are determined, the analysis is termed proximate.
In the second case, where elements are determined, the analysis is termed ultimate.
For convenience, quantitative methods may be classified thus:
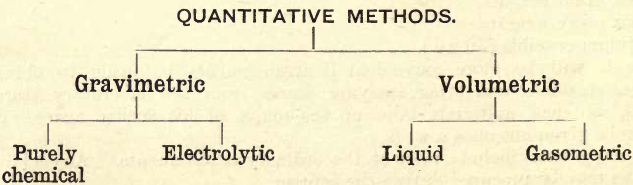
GRAVIMETRIC METHODS
Purely Chemical
In this section certain gravimetric methods—those of fire-assaying -are omitted. These, on account both of their nature and importance to the metallurgical chemist, are dealt with in a special section in Part III.
Conditions.—As a general rule, in the methods to be given the following conditions hold:
a. The element to be estimated is generally converted into some compound.
b. This compound is insoluble in the liquid medium present.
c. This compound has a definite, known composition.
d. This compound can be brought into a state suitable for accurate weighing.
Processes.—Though variations in treatment, involving evaporation, digestion, distillation, and other processes, are met with, it may be said that generally the following processes are necessary when estimating an element or elements by “ purely chemical ” gravimetric methods.
- Sampling and preparing the substance for solution.
- Weighing the quantity taken for analysis.
- Solution of the substance.
- Precipitation of the element sought.
- Filtration of the precipitate thus obtained.
- Washing the precipitate.
- Drying, or drying and Ignition
- Weighing the precipitate.
- Calculating the result.
These operations will be briefly described in turn; and before proceeding to the first estimation given, the student must carefully read through these descriptions, obtain the necessary apparatus and reagents, and check the accuracy of the balance and weights to be used. He must also in every analysis consider the purity or otherwise of the reagents used. This point has already been referred to under “ qualitative analysis,” and will again be briefly noticed under “ miscellaneous instructions.”
Sampling
The Preparation of the Substance for Solution. — Too much emphasis cannot be laid on the fact that without careful sampling the value of an estimation is practically nil. In the majority of cases the chemist is required to determine the percentage of an element or compound present in a considerable mass of the substance given, and (except in the case of chemical salts) it is the safe course to assume that the element to be estimated is distributed unevenly throughout the mass. Therefore, to accurately estimate the quantity of the element present in the whole mass, an average sample must be obtained. The sample taken for analysis must have been so taken that it represents the true average contents of the mass.
Discrepancies in the results of different chemists are more frequently due to errors in sampling than to errors of method and manipulation in the analysis itself. When, for example, the student is given a 10 to 15 gram sample of galena, and is instructed to weigh about .5 gram for analysis; then, if he wish the results of several consecutive analyses to agree, he must be absolutely certain that, to the best of his ability, he has so selected this .5 gram lot, that it represents the true average contents of the larger sample.
It is useless for the student to worry his brain searching for defects in his analytic manipulation, when results do not agree, unless he is perfectly sure that the sampling error cannot account for the discrepancies.
The sole conditions of success are that the best methods and manipulation are preceded by accurate sampling.
The substance to be sampled may be either a Solid, Liquid, or Gas.
Solids
The solid presented for analysis may vary from a few ounces to several tons. In this chapter large parcels will not be considered; not that their sampling is unimportant or easy. The reverse is the case. This matter will be considered in the section devoted to Assaying. Here it is assumed that the student is presented with a few ounces of the material either in coarse lumps or as a fine powder.
Sampling small Parcels
When in the form of coarse lumps reduce the whole sample by pulverising in a cast-iron mortar such as is used in the reduction of ores for assaying. If, however, the substance has chemical action on iron, or is so hard that particles of iron from the mortar become mixed with the sample, special methods of reduction must be resorted to. To avoid chemical reactions occurring, mortars of porcelain or agate must be used, the latter when the substance is hard. To avoid contamination by particles of iron a large “diamond mortar,” such as is shown in fig. 37, is used.
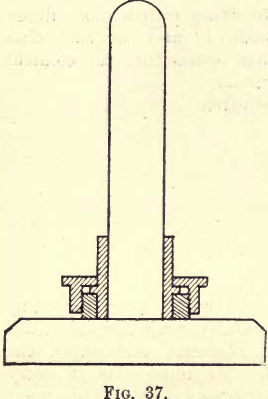
This mortar is made of steel “ case-hardened,” and will be found satisfactory in most cases. Its use is self-evident. The piston being removed, a few small pieces of the solid are inserted, the piston replaced and a few blows smartly struck with a hammer. The powdered solid is then removed and placed on a clean sheet of glazed paper. If the lumps of the solid are too large for the mortar, wrap them one at a time in clean paper; place on a clean anvil and crush with a few blows of a heavy hammer. When the sample has been thus reduced pass the fine material through a 30 sieve. Any particles not passing through the sieve are re-crushed until of the requisite fineness.
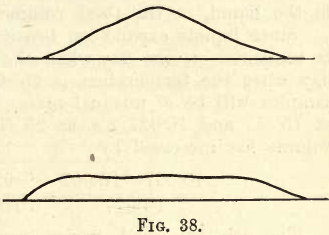
No hard and fast rules can be laid down regarding the reduction of various materials. In every case the student must consider first the chemical nature of the solid—i.e., as regards its chemical effect on the reducing appliances; then he must consider its hardness and the probability of mechanical contamination. Generally for chemical salts the Wedgwood mortar may be used; for soft minerals (not harder than 6 Moh’s scale) the iron mortar or “ bucking plate ” (see Assaying); and for harder minerals, etc., the diamond-mortar, though even then there is danger of slight mechanical contamination. The student now has 20 or 30 grams of the solid in a state of fine division. With the “ Spatula ” (a spatula with a 10 cm. steel blade will be suitable unless chemically acted on by the powder, in which case use vulcanite or platinum), carefully mix the powder by repeatedly turning it over, flattening it out and heaping it up. When thoroughly mixed (a few minutes should suffice), flatten out the heap into the form of a shallow truncated cone. See figs. 38 and 39.
With the spatula divide the heap into quarters as shown. Remove two diagonally opposite quarters. Again mix the remainder and re-quarter as before, till finally by repetition of this process about 5 to 10 grams remain.
If the student is instructed to take from .5 to 2 grams of the material for each analysis he will have sufficient material for five to ten estimations. If a larger quantity is required for each estimation the sample must be proportionately larger. To obtain some idea of the weight of the sample the student may roughly weigh it on a set of “ pulp-scales ” (see Assaying).
This sample has now to be ground to such a state of fineness that it is easily soluble in the water, acids, fusion-mixture or other solvent medium to be used. This grinding is almost invariably performed in the agate mortar. As a rule, chemical salts require little additional grinding. Mineral powders, however, generally require prolonged treatment in the agate mortar, which for this propose should be at least 9 cm. in diameter. With smaller mortars progress is painfully slow. The grinding is performed most satisfactorily by placing in the mortar about half a gram of the solid, and then the powder is ground by a circular grinding motion of the pestle, assisted by fair pressure of the hand. This grinding must be continued till on rubbing a small pinch of the powder between the tips of the lingers no grit is felt. Then proceed with another portion, and so on till sufficient is ground for five or six analyses. The finely ground sample is now spread out on a small sheet (20 cm. x 20 cm.) of glazed paper. It is then carefully mixed and flattened out. The portion for analysis is then taken either by systematically dipping with the point of the spatula or by running through the flat-heap small trenches with the edge of the spatula.

The student will find that the thorough reduction of minerals is the most tedious part of the whole analytical process. Though in some cases the process is exceedingly laborious, he must resist every inclination to shirk the thorough reduction of the portion for analysis. In the majority of cases the time and labour required are not excessive, and in the few cases where they appear so it must be remembered that accurate results cannot be obtained otherwise. Of late years power-driven agate mortars have been successfully introduced in technical and other laboratories.
Liquids
In most cases the samples presented for analysis are solids. Sometimes, however, the student is asked to determine the quantity of some element or compound present in a liquid. For instance, he may be asked to determine the SO3 present in a Winchester quart bottle partly filled with H2SO4. An average sample can be quickly obtained by shaking the bottle and pouring out the desired quantity. This portion for analysis may be either measured or weighed. It may be measured either from an accurate “ pipette ” or “ burette ” (see Volumetric Analysis); but either the temperature of the solution must be carefully noted by immersing a thermometer in the liquid, or the total volume of the solution must be ascertained.
Since liquids expand on heating, it will be seen that if 10 c.c. of water be measured on one day when the temperature is 15° C., and on the following day when the temperature is 25° C., another 10 c.c. lot be measured, the two samples will be of unequal mass 10 c.c. water at 0° C. become 10.007 c.c. at 15° C. and 10.027 c.c. at 25° C.; that is, between 15° C. and 25° C. the volume has increased by
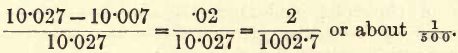
The density, which varies inversely as the volume has decreased, therefore, assuming that the acid expands similarly, the sample of 10 c.c. measured at 25° C. contains 1/500 less SO3 than the portion measured at 15° C.; that is an error of 0.2 per cent., assuming the solution expands similarly to water.
This matter will be dealt with more fully under “Volumetric Analysis.”
If, however, the contents of the bottle be measured in an accurate “ Test Measure” (see “Volumetric Analysis”), and are found to be, say, 1600 c.c., and 10 c.c. be taken at the same temperature, the estimation can be made accurately, provided these measurements be repeated on taking each fresh portion. This method, however, is of little practical use, on account of the difficulty of accurately measuring such a large quantity.
The student will obtain the best results by carefully weighing a small beaker or flask, and then running in a suitable quantity of the liquid, and then re-weighing the vessel and contents. The difference gives the weight of liquid taken.
The first method gives the results as so many gms. SO3 in so many c.cs. of the liquid ; the last-mentioned method as so many gms. SO3 in so many gms. of the liquid. To convert c.cs. to gms. the density must be known at the temperature of measurement. Examples of the necessary calculations will be given later on.
The metallurgical chemist is rarely required to sample semi-solid substances such as butter, lard, etc. He may, however, be required to analyse either moist or hygroscopic substances. With moist substances a boring rod (cheese sampler) may be used to obtain an average sample. In this the moisture is determined (see Assaying), and the dry sample is assayed as usual. Certain hygroscopic substances present some difficulty, as they rapidly absorb moisture from the air. With these quickly pick from the containing jar an average sample, weighing it quickly and accurately on the pulp scales. It may then be dissolved in water, and treated as a liquid.
Gases
The methods of collecting these are described or referred to in the section on Technical Analysis, and the student is there referred to certain authorities on this subject. In this section the subject is not touched on.
Weighing the Substance
In gravimetric analysis the method of measurement adopted is that of weighing—one of the most accurate means we possess—and by it the student may with ease and rapidity weigh objects of 100 gms. accurately to .001 gm., that is, with an error of less than 1/100,000 Or again he may weigh 1 gm. accurately to .0001 gm., an error of less than 1/100,000.
Before proceeding to describe the operation of weighing, the various methods of weighing, and the selection of a suitable weight of the substance, a description of the balance, weights, and accessories is necessary.
https://www.911metallurgist.com/analytical-balance
