Table of Contents
- Raw Materials
- Experimental Program
- Separation Between Chromium and Iron
- Separation Between Chromium and Cobalt and Nickel
- Separation Between Chromium and Other Alloying Elements
- Reduction of Chromium-Bearing Slags
- Reduction With Other Reducing Agents
- Appendix.—Analytical Methods
- Sample preparation of spectroscopic analysis
The experimental program described in this report demonstrated key process steps for the recovery of chromium as ferrochromium in a batch process. The process involves oxidation of chromium into a slag phase, subsequent separation of the slag phase from the remaining metal, followed by reduction of chromium from the slag phase to produce ferrochromium.
Although world chromium resources are ample for the foreseeable future, political and economic events have raised doubts about the uninterruped availability and reliability of chromium supply. Chromium is used extensively in the metallurgical industry and has no technically viable substitute for such critical applications as nickel-base superalloys required for aircraft gas turbine engines.
The United States is now almost totally dependent on foreign resources for its chromium mineral needs although it does derive a portion of its metallic chromium supply through recycling of scrap materials. A large quantity of chromium in alloy scrap, primarily stainless steel, is currently being recycled. However, a large amount of chromium in scrap is not recycled domestically and hence, is lost to the industry. Some of this scrap is downgraded into lower value applications where the chromium is either lost in processing or could be replaced by less critical elements. Most chromium-bearing scrap now recycled or refined to recover more valuable elements such as nickel, cobalt, and molybdenum, has significant amounts of chromium that are incompletely recovered.
The technology does not now exist to economically separate chromium from complex Ni-Fe-Cr alloys. Such a process would provide chromium values which could be used in times of national emergency. The Federal Emergency Management Agency (formerly the Federal Preparedness Agency) sponsored this research through the Bureau of Mines, U.S. Department of the Interior, to determine the feasibility of using a pyrometallurgical approach involving the oxidation of chromium to a slag phase, separation of the slag, followed by reduction of chromium from the slag phase.
This report supplements a two-part report published as Bureau of Mines Information Circulars describing the results of two surveys that examined the availability of superalloy scrap, cast high-alloy chromium-bearing scrap, and stainless steel scrap. Information presented includes identification of the types of scrap, sources, quantities, and nature of ultimate disposition, such as direct recycling, intermediate refining, or disposal.
Raw Materials
As a primary requirement of this developmental program, the selected process should be capable of recovering chromium from superalloy scrap. A superalloy is defined as an alloy developed for high-temperature service where relatively high stresses (tensile, thermal, vibratory, and shock) are encountered. Since the term superalloy encompasses a wide variety of alloy designations, the process must necessarily be capable of handling a wide variety of metallic scrap materials.
In a parallel program, a two-part survey was conducted to assess and confirm the domestic availability of metallic scrap containing chromium.
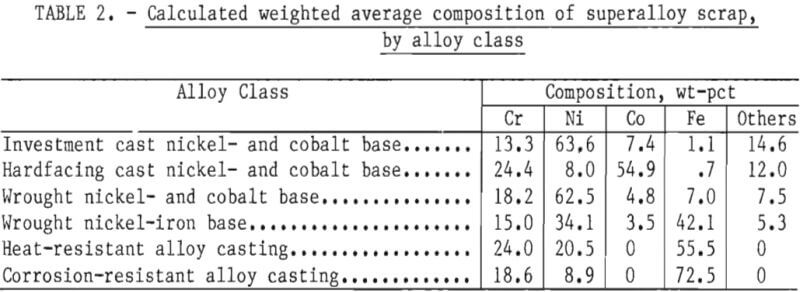
Part 1 dealt with superalloy scrap, while Part 2 concentrated on stainless steel scrap. Survey results indicated that in addition to stainless steel scrap, wrought nickel- and cobalt-base superalloys and wrought nickel-iron-base superalloys represent major current and projected sources of obsolete scrap. Alloy classes with even greater quantities of obsolete scrap are the cast heat- and corrosion-resistant alloys, which are primarily iron-base alloys with chromium and nickel. The quantities of obsolete superalloy scrap, as estimated in Part 1, are given in table 1. The calculated weighted average composition of these scrap types are given in table 2. These two tables, taken collectively, indicate the quantities and general compositions of raw materials that the selected process must be capable of handling if it is to serve as a domestic source of chromium units from superalloy scrap.
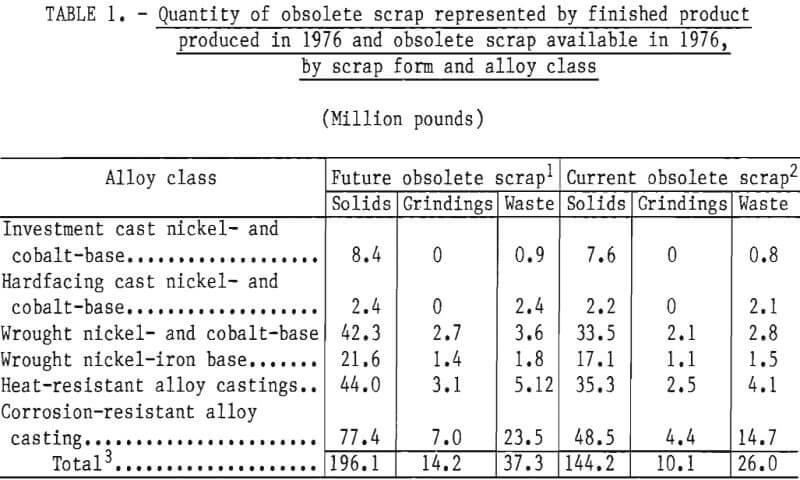
Examination of table 1 shows that the four major superalloy classes account for more than 90 pct of the superalloy scrap. These four classes can be chemically classified into low- and high-iron alloys: Low-iron alloys included wrought nickel- and cobalt-base alloys having an average iron content of about 7 pct iron. High-iron alloys include wrought nickel-iron-base alloys having a weighted average of 42 pct iron, heat-resistant alloy castings having a weighted average of 55.5 pct iron, and corrosion-resistant alloy castings having a weighted average of 72.5 pct iron.
The results of the survey reported in Part 2 showed that an estimated 369,000 tons of stainless steel scrap were not being recycled or being downgraded. This amounts to about 62,000 tons of contained chromium based on an average chromium content in stainless steel of 16.7 pct. In the commonly found types of stainless steel, nickel is present in concentrations of 5 to 15 pct while iron is typically in the range of 60 to 80 pct. Thus, the chromium, nickel, and iron concentrations found in stainless steel are not too dissimilar to the predominant corrosion resistant superalloy castings shown in tables 1 and 2.
General Approaches
Several general types of unit processing techniques can be considered for the recovery of chromium from superalloy scrap:
Pyrometallurgical-based operations often involving a melting step. Such processes can be based on selective oxidation and reduction as described by Kenworthy; Barnard; and Filar; selective chlorination as reported by de Beauchamp, and processes based on sulfide or carbide affinities.
Electrochemical or electrolytic processes in which an electric current is used as an aid in refining, dissolving, or plating chemical species from solutions or melts; such as the work reported by Kenworthy and Nagai.
Hydrometallurgical processes based largely on wet chemical methods. Approaches include leaching with hydrochloric acid or chlorine, ammonia leaching, and caustic fusion followed by leaching.
Miscellaneous techniques not easily classified, such as removing nickel from superalloys by nickel carbonyl.
Selected Approach
After giving due consideration to various approaches, a pyrometallurgical oxidation-reduction approach was chosen because of technical soundness, limited number of process steps, energy efficiency, environmental soundness, and simplicity—chromium is separated from the major superalloy constituents early in the process; recovery of other elements is potentially feasible.
Kenworthy demonstrated it is feasible to separate chromium from nickel and cobalt in superalloy scrap by selective oxidation of chromium into a chromia-rich slag. His experiments showed that it was possible to oxidize chromium, columbium (niobium), and some of the iron into the slag phase while retaining nickel, cobalt, and residual iron in the metallic phase.
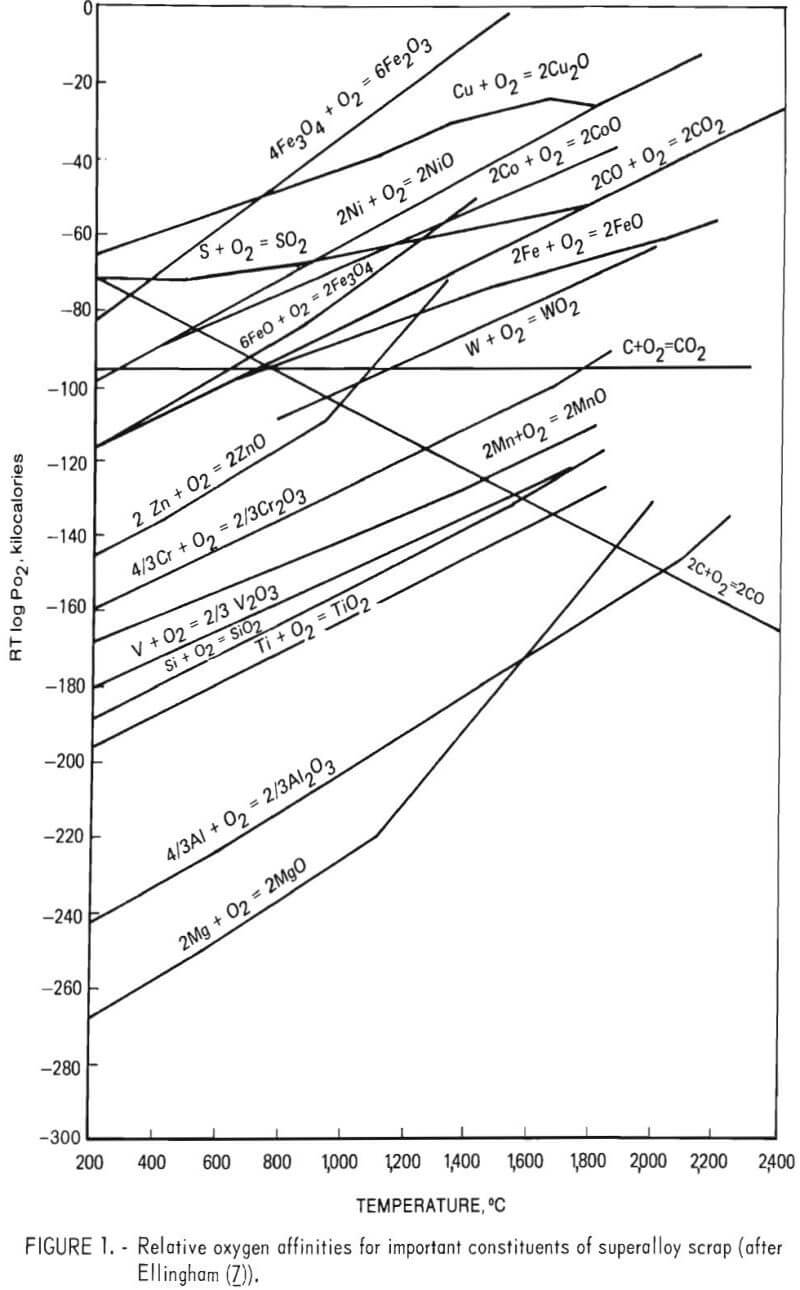
Kenworthy’s results would be expected from thermodynamic considerations. Figure 1 shows the relative oxygen affinities for many important constituents of superalloy scrap. As indicated, the tendency for nickel, cobalt, and iron to combine with oxygen is less than that of chromium. Given a sufficiently high oxygen partial pressure and neglecting activity coefficients, one would expect chromium to be oxidized out of a molten bath of superalloy scrap prior to the removal of nickel, cobalt, and iron under equilibrium conditions. Furthermore, these thermodynamic data show that by carefully controlling oxygen partial pressure, oxidation of cobalt, nickel, and iron could be prevented completely, while providing a chromium-bearing slag phase.
The chromium-rich slag phase could be considered a chromite ore suitable for subsequent smelting to produce high- or low-carbon ferrochromium. After separation from chromium-depleted metal, the molten slag phase could be reduced by a variety of means, such as with ferrosilicon, scrap alumium, or carbon.
Barnard and Filar have shown that ferrosilicon reduction will easily recover over 90 pct of the chromium in the slag. Furthermore, ferrosilicon reduction has been commonly used in stainless steel production to control chromium losses to the slag. Good slag-metal contact is necessary since chromium reduction from the slag appears to be diffusion-controlled as found by Filar. Again figure 1 shows that, under equilibrium conditions, the silicon in ferrosilicon would be expected to reduce chromium but not aluminum or titanium.
Based on this previous work, it is proposed that chromium be recovered from the other major alloying elements in superalloy scrap, namely nickel, cobalt, and iron by the selective oxidation of chromium into a chromium-rich slag (as shown in fig. 2). After separating chromium depleted metal from the slag, the second step of the process would involve slag reduction, wherein chromium oxide in the slag would be reduced with carbon, aluminum, or ferrosilicon to produce a ferrochromium product.
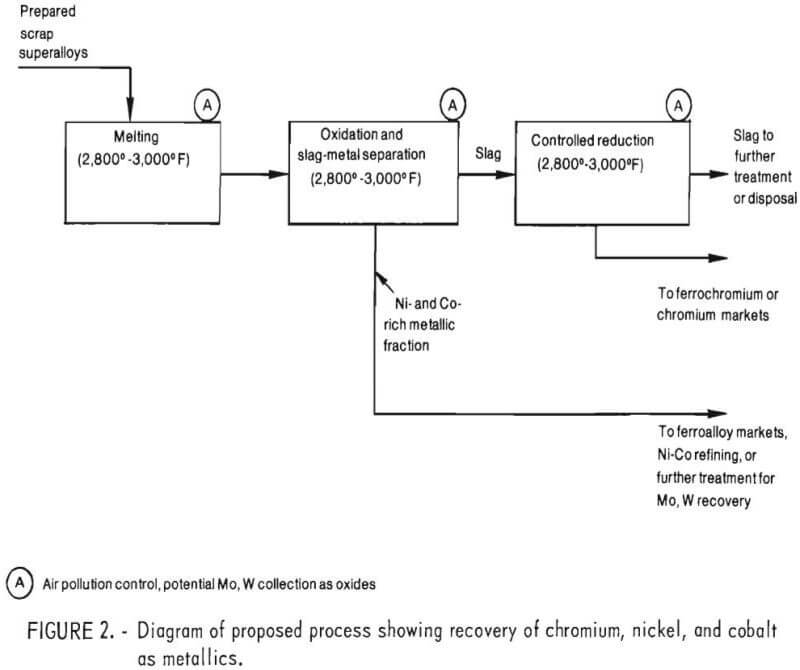
With regard to ferrochromium, steelmakers have generally preferred high chromium-to-iron ratios. However, discussions with steelmakers indicate that they would consider using ferrochromium with a Cr-Fe ratio of one. Similarly, discussions with scrap dealers indicate that they would consider purchases of any nickel-cobalt bearing alloys. Thus, outside of possible contamination of product ferrochromium by minor alloying elements present in superalloy scrap feedstocks and process economic consideration (issues which were beyond the scope of this investigation), there appears to be no major difficulties in marketing the products and byproducts produced by this proposed process.
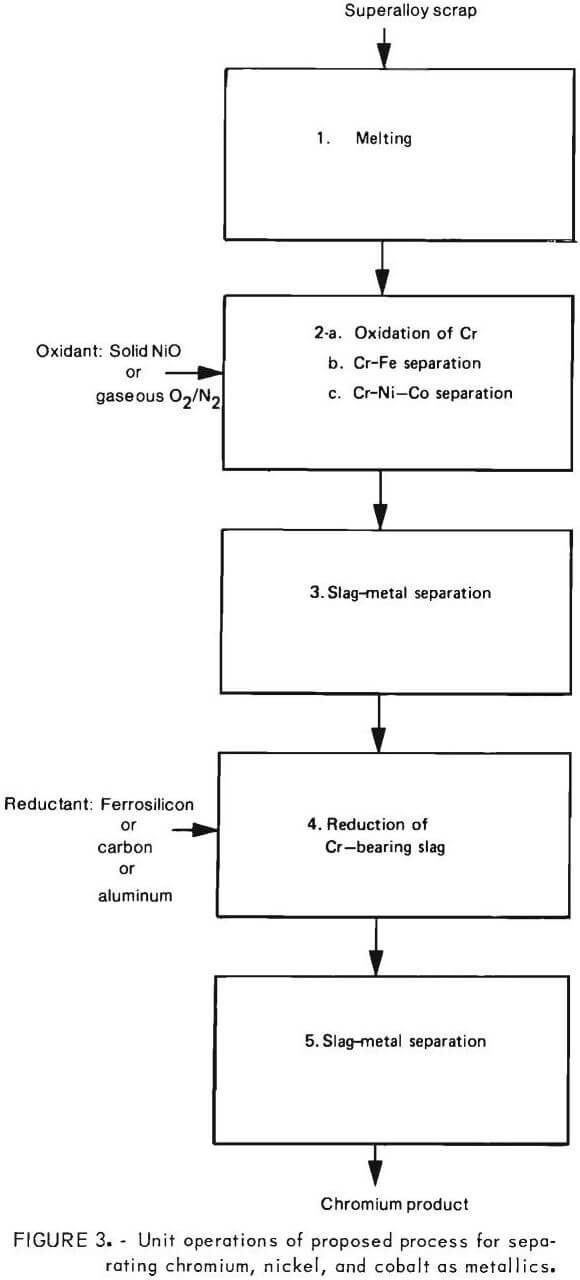
Experimental Program
The purpose of the experimental program was two fold:
- Establish a flowsheet for a process capable of separating and recovering chromium from superalloy scrap.
- Obtain necessary data for the preliminary design of a demonstration pilot plant capable of treating an average of 100 pounds per hour of scrap to recover a minimum of 65 pct of the contained chromium in a state sufficiently pure to provide a marketable product.
Key process steps needing demonstration are shown in figure 3. Of these, the selective oxidation of chromium into a slag phase (step 2) and reduction of chromium from a chromium bearing slag into a metal phase (step 4) are of particular importance. Hence, the main thrust of the experimental program was directed at verifying these two steps.
Metal Samples
Four types of superalloy scrap, listed in table 3, were tested in this program. These types were selected because they represent each of the major types of superalloys currently being produced.
Samples of superalloy scrap were obtained from the INCO Research Center and a local scrap dealer. The samples were obtained as pieces in various physical configurations. In general, pieces weighed less than 0.25 pound. Chips of metal were removed from each type of scrap and chemically analyzed to insure the scrap’s identity. Chemical compositions are given in table 3.
Scrap samples were not prepared in any special manner prior to being used. Large pieces were cut, as necessary, to fit within the furnace crucible. Gross surface impurities, such as dirt and grease, were removed by wiping. However, scrap pieces were not subjected to a complete chemical cleaning, such as washing with solvents or polishing.
In addition to superalloy scrap, several experiments were performed with low-cost AISI 430 stainless steel occasionally mixed with carbon steel. These metal samples, used to demonstrate the feasibility of chromium oxidation in the presence of large amounts of iron, were prepared in a similar manner to superalloy scrap samples.
Other Charge Materials
Besides metal samples, other materials charged to the furnaces included fluxing agents and reductants. Reagent-grade silica and lime and NyCor Wollastanite (Interpace Corp.) were used as fluxes. Throughout the experimental program, a silica-to-lime ratio of 2:1 (by weight) was used. Fluxes were prepared by mixing silica and lime or Wollastanite in the proper proportions followed by sintering in an open flame to produce 1/8- to ¼-inch- diameter pellets. This was done to facilitate flux additions in the presence of a sweeping inert gas. Later in the program, powdered fluxes were simply poured onto molten baths without prior sintering when it was found that losses caused by the sweeping gas were insignificant.
Three kinds of reductants were tested for reducing chromium from chromium oxide-bearing slags—aluminum, carbon, and ferrosilicon. Aluminum and carbon were high purity materials in fine powdered form. Ferrosilicon pieces, also of high purity (75 wt-pct Si), ranged from 1/8 to 1 inch in diameter. Reductants were either poured onto the surface of a molten slag layer or were mixed with a granulated slag followed by meltdown.
Apparatus
Two induction furnaces were used in the experimental program: A 50-kW furnace with a molten charge capacity of about 20 pounds and a 20-kW furnace with a capacity of less than 1 pound. The large furnace was used for both oxidation and reduction experiments, whereas the small furnace was used solely for reduction experiments.
50-kW Furnace
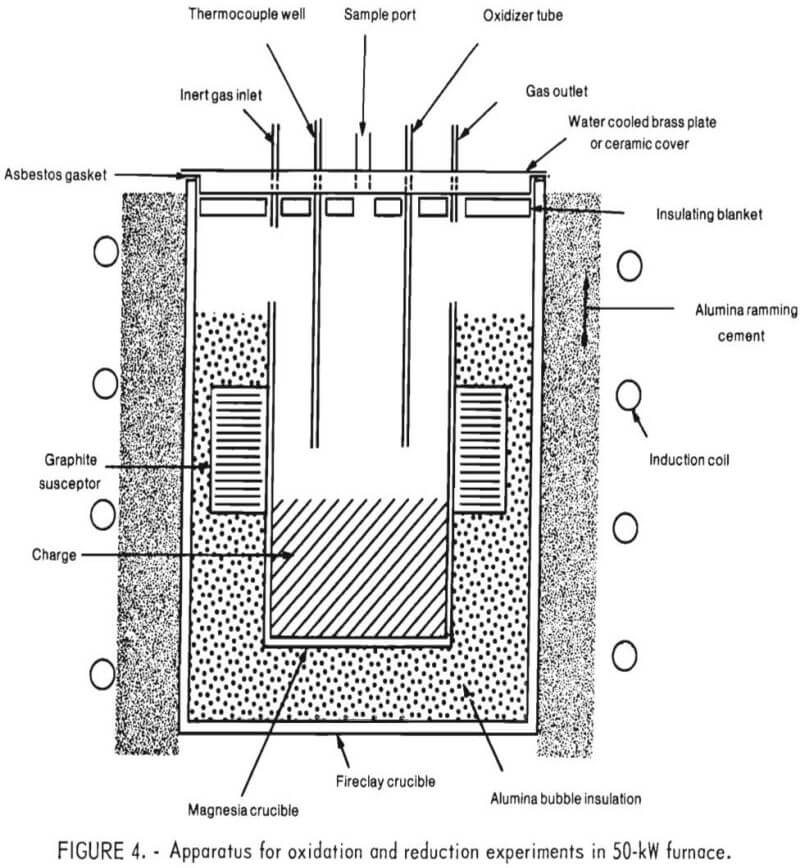
Figure 4 is a schematic representation of the crucible arrangement within the 50-kW induction furnace, which was powered by a variable frequency motor generator. As shown, a 12-inch-diameter induction coil was coupled directly to the metal charge. The charge was contained in a magnesia crucible (approximately 4½-inch ID, 8 inches high) that was placed within a fireclay crucible. A ½-inch-thick graphite susceptor surrounded the inner crucible near its center to provide additional heat to the slag and prevent it from freezing.
The space between the two crucibles was filled with alumina bubble insulation. The space between the induction coil and the outer crucible was packed with alumina ramming mix. Oxidizing gases (air and/or oxygen) were introduced either through a lance inserted through the top cover or through a single tuyere in the bottom of the furnace.
Initially, an airtight apparatus was desired to prevent metal oxidation during meltdown. To accomplish this, the rim of the inner crucible was machined to accommodate an asbestos gasket over which was placed a water-cooled brass plate. A 1-inch-thick ceramic fiber insulation blanket was placed below the brass plate, to serve as insulation as well as a radiation shield. Pressure measurements, taken later in the program, indicated a positive pressure within the crucible during the injection of inert or oxidizing gases. Hence, an airtight apparatus was unnecessary and a cover, fabricated out of castable refractory material, was used in all successive experiments.
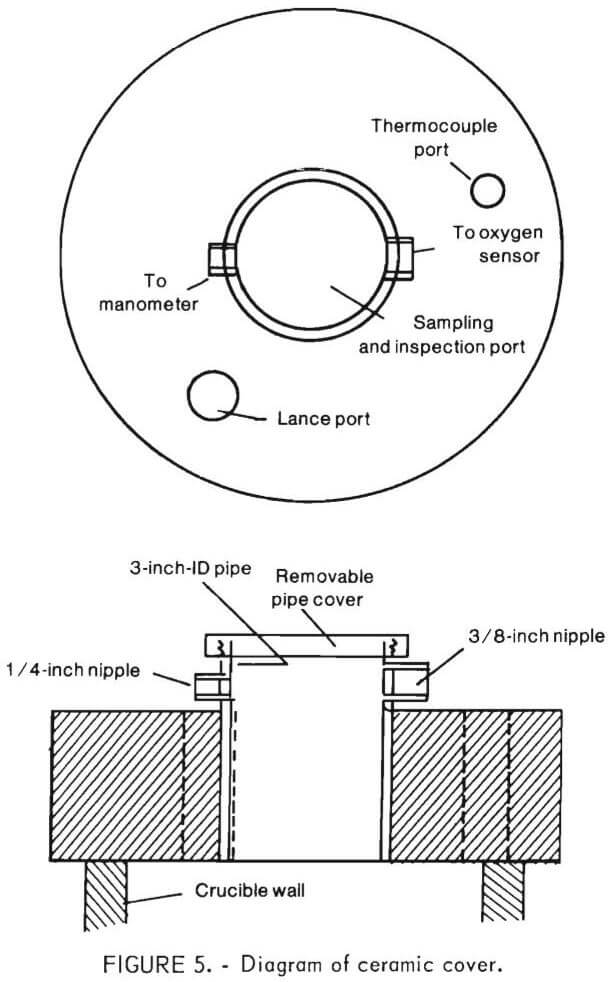
A diagram of the castable refractory (ceramic) cover is shown in figure 5. Figure 6 shows the ceramic cover in place above the crucible. For insulation, a ¼-inch asbestos layer was used with the ceramic cover rather than the ceramic fiber blanket.
Both ceramic and brass tops had provisions for a sampler tube, off-gas exit tube, a port for pressure measurements, and a thermocouple well. For top-blowing oxidation experiments, an inert gas inlet and an oxidizing gas inlet were provided. These ports were unnecessary (and therefore sealed off) for bottom-blowing experiments since oxidizing and inert gases were injected into the bottom of the inner crucible.
The apparatus was instrumented as follows. Temperature measurements were taken with a platinum/platinum-rhodium thermocouple placed above the melt. These measurements were supplemented by optical pyrometer readings taken by sighting through the sampling port. The oxygen content of crucible off-gas was monitored on a periodic basis with an oxygen probe. The probe was a polarographic instrument with a pinole diffusion barrier rather than a membrane to minimize temperature sensitivity. Gas pressure within the crucible was measured with a small-diameter liquid manometer. Gas injection rates into the crucible were measured with rotameters and pressure gages.
The experimental aparatus for bottom blowing through a tuyere is of importance because it is rarely done on such a small scale. A diagram of the fail-safe gas delivery system is shown in figure 7. The system was designed so that a reasonable flow of gas could be maintained into the bottom of the crucible at all times. It consisted of two nitrogen tanks feeding into a single line, past a rotameter and a pressure regulating valve into a 3/16-inch ceramic tube that was rammed into the center of the crucible bottom. All gas-lines were copper 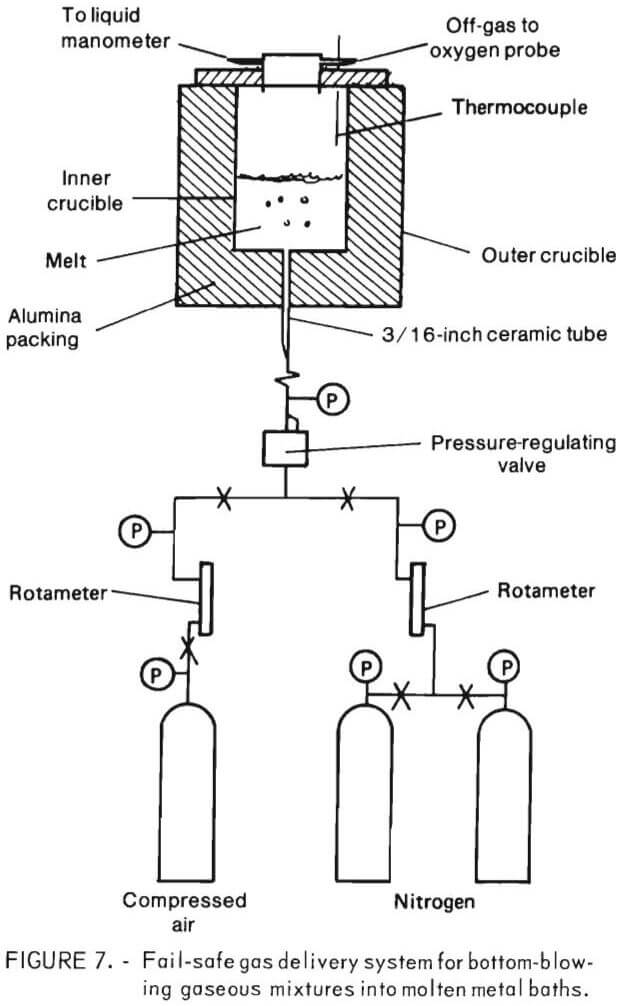 tubing with swaged fittings, except the line from the final pressure gage to the ceramic tube, which was high-pressure rubber tubing. Compressed air, after passing through a rotameter, entered the feedline upstream of the pressure regulating valve. Compressed air was set at a slightly higher pressure than nitrogen so that once air was turned on, any nitrogen flow would be
tubing with swaged fittings, except the line from the final pressure gage to the ceramic tube, which was high-pressure rubber tubing. Compressed air, after passing through a rotameter, entered the feedline upstream of the pressure regulating valve. Compressed air was set at a slightly higher pressure than nitrogen so that once air was turned on, any nitrogen flow would be
automatically stopped. Should the airflow be interrupted at any time, nitrogen would begin flowing instantaneously. Thus, the only way that gas flow could be completely stopped, which would instantly terminate an experiment by allowing molten metal to empty out of the crucible via the ceramic tube, would be if both nitrogen tanks emptied. Nitrogen was injected into the lines at approximately 36 psig, compressed air at 42 psig, and the pressure regulating valve was set to provide a gas flow at about 5 psig. As such, pressure fluctuations within the crucible did not disturb rotameter readings.
20-kW Furnace
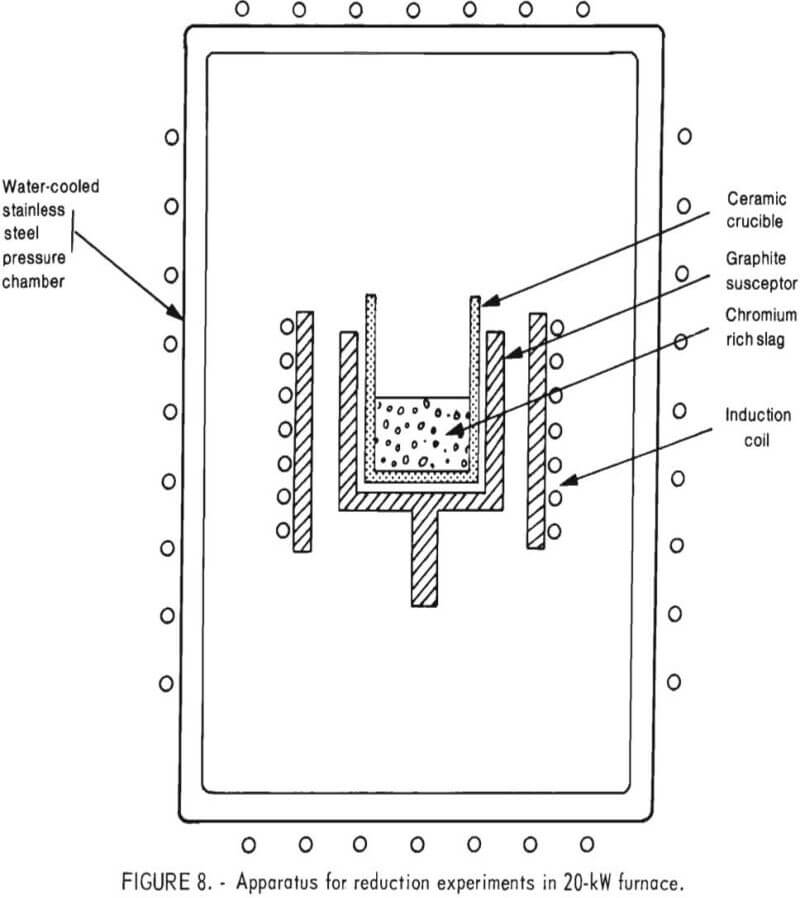
The 20-kW induction furnace operated at radio frequencies to inductively heat a small-sized graphite shell (susceptor). The crucible arrangement, located within a stainless steel pressure chamber, is shown
schematically in figure 8.
As indicated, a 3-inch-diameter induction coil was coupled to a graphite susceptor. Inside the susceptor was a refractory crucible containing the charge (chromium oxide-bearing slag and reductant). Although some magnesia crucibles were used in these 20-kW furnace tests, the majority of reduction experiments were performed in alumina crucibles. A ceramic fiber blanket was placed between the susceptor and induction coil to serve as insulation as well as a radiation shield.
Method
Oxidation experiments were performed by top blowing oxidizing gases onto the surface of molten baths and by bottom blowing gases through the baths. The experimental procedure was identical for both cases. In addition, one oxidation experiment was performed by mixing known amounts of solid nickel oxide and AISI 430 stainless steel that were charged to the 50-kW furnace. After meltdown, sufficient time was allowed for equilibration of the nickel oxide with chromium to produce chromium oxide and metallic nickel.
Reduction experiments were performed by adding the reductants onto the surface of chromium-bearing slags in the 50-kW furnace and by mixing reducing agents with synthetic solid chromium-bearing slags, followed by charging the mixture to the 20-kW furnace. The experimental procedure was somewhat different for these two cases. Detailed procedures for both oxidation and reduction experiments are described.
Oxidation Experiments
Scrap samples were weighed and charged to the inner crucible. The asbestos gasket was placed over the flat rim of the outer crucible followed by a ceramic fiber insulation blanket and the water-cooled brass lid, or simply the ceramic cover. To prevent off-gas leakage around the ceramic cover, the cover was sealed in place with castable refractory material, which was allowed several hours to completely cure. The thermocouple was placed in the thermocouple well, and gas connections and water connections (if necessary) were made.
The crucible was sufficiently purged with inert gas to insure that oxygen would not be present during meltdown. Three kinds of inert gas were used: nitrogen, argon and an argon-5 pct hydrogen mixture. Inert gas was allowed to flow throughout the meltdown period. Furnace power was turned on at about 3 kW. Temperature measurements were taken as the charge heated. Power settings were increased every 15 to 20 minutes up to a setting of 12 to 14 kW when meltdown was achieved. This prolonged melt procedure was followed to reduce thermal shocks to the ceramic crucible.
Once the charge was completely molten, large thin pieces of scrap were added through the sampling port as necessary. Following the meltdown of this material, the bath was allowed to stand for about 15 minutes with the power at a reduced level. A sample of the melt was then taken.
https://www.911metallurgist.com/process-recovering-chromium-metals-scrap/
Samples were taken by two methods. In general, a sampling tube, consisting of a 3-mm-ID, thick-walled quartz tube, with a rubber squeeze bulb, was used. With the bulb in the deflated position, the tube was quickly lowered into the bath. Pressure on the squeeze bulb was released, drawing molten metal into the tube. The sample was then quenched in cold water, causing the quartz tube to break off. Alternatively, samples were taken by dipping a piece of stainless steel flat stock, bent at 90° near its end, into the melt. Portions of the bath froze to the bar, which was then removed from the crucible. After quenching, the sample was removed from the bar by a sharp blow with a hammer. Metal samples were obtained with both methods; slag samples could be taken only with the latter. All samples were chemically analyzed for weight-percent chromium, cobalt, nickel, and iron. A description of analytical techniques is given in the appendix.
In general, metal samples obtained by these methods were reasonable; that is to say chemical analyses of metal samples showed reproducible patterns with oxidation or reduction equilibration time. However, the reliability of slag samples was questionable. This appeared to be due to the difficulty of obtaining slag samples that were not contaminated with metal phase. Because of the highly viscous and, at times, almost frozen nature of slags within the 50-kW induction furnace, it was extremely difficult to sample only the slag phase. To alleviate this problem, a graphite susceptor was installed to provide additional heating on the premise that a less viscous slag would simplify sampling procedures. However, it was found that the susceptor did not provide sufficient heat to guarantee liquid slags. Hence, chromium oxidation was followed by tracking the chromium content of metal samples. Selected slag samples were used to verify the results.
After the initial sample was taken, flux was added and allowed to melt. Inert gas flow was terminated and oxidizing gas flow begun. Periodically during the blow, metal and slag samples were taken, bath temperature monitored, and oxygen utilization determined. Power adjustments were made as necessary to hold the bath at about 1,600° C. During certain experiments, additional flux additions were made to provide a more fluid slag. Once the desired amount of oxygen had been injected into the crucible, either by top or bottom blowing, gas flow was switched over to inert gas, and the melt was allowed to stand for 15 to 30 minutes, after which the final metal sample was taken. The melt was then either cast (by pouring into a sand mold) or left to cool in the vessel. Alternatively, the melt was used as starting material for a reduction experiment.
Reduction Experiments
The experimental procedure for reduction experiments performed in the 50-kW furnace was to add a weighed amount of reductant (graphite, ferrosilicon, or aluminum) onto the surface of a molten chromium-bearing slag. The slag and associated metal phase had been produced during an oxidation experiment. Samples of both were taken prior to the addition of reducing agents.
In general, the effect of reductant on the system was rapid. Samples of the metal and slag phases were taken periodically up to 1 hour after addition of reductant. After a final set of samples was obtained, the melt was cast. Throughout the experiment, the crucible was purged with inert gas. Bath temperature was monitored with optical pyrometer readings and power adjustments were made as necessary. As with oxidation experiments, an attempt was made to keep the bath as close to 1,600° C as possible. Actual temperature readings ranged from 1,550° to 1,650° C.
The experimental procedure for reduction experiments performed in the 20-kW furnace was as follows. The charge, consisting of chromium oxide, lime-silica flux, and reductant, and the crucible were weighed. The charge was transferred to the crucible which was placed within the graphite susceptor. The ports on the stainless steel enclosure were sealed and the cavity filled with inert gas. The power was then turned on. Temperature measurements were made with an optical pyrometer. The charge was allowed to melt and held molten for periods of time ranging from 15 to 45 minutes after which power was shut off. After the crucible had cooled, the contents were removed, and metal and slag portions were separated, weighed, and sent for analysis.
Results and Discussions
Experiments were designed to verify the technical feasibility of major process steps and to provide basic engineering data for the preliminary design of a pilot plant. Experimental results are therefore presented according to major process steps in this process. As stated previously, since oxidation of chromium and its subsequent reduction represent critical steps, the main thrust of the experimental program was directed at these two steps.
Scrap Melting
Open arc electrical furnaces have been widely used for scrap melting in carbon and stainless steel industrial practice. In this experimental program, an induction furnace was utilized since it is difficult to undertake open-arc-furnace testing for scrap melting and partial oxidation on a laboratory scale.
The results of scrap melting experiments, performed as a necessary preliminary to oxidation experiments, indicated that the packing density of the scrap charge largely determines the ease with which melting can be achieved. Thin sections of WI-52 and B-1900 superalloy scrap, loosely packed inside the crucible, could not be melted. However, if the scrap was cut and pressed into compact pieces to achieve high packing densities, satisfactory melting could be achieved. Furthermore, melting times could be significantly reduced by adding scrap to a molten heel. This result is consistent with industrial electric arc furnace operations.
The experimental results pertaining to melting are not directly applicable to pilot-plant design since an electric arc furnace was selected for pilot-plant experimentation. Discussions with steelmakers and electric arc furnace suppliers indicate that no unusual technical problems associated with scrap melting should arise.
Chromium Oxidation
Oxidation experiments were performed by top blowing oxidizing gases (mixtures of oxygen and nitrogen) onto the surface of molten metal baths, and by bottom blowing compressed air through molten baths. Bottom-blowing experiments were performed in order to demonstrate whether better oxygen utilization could be achieved than with top-blowing experiments. Forty-two oxidation experiments were performed. Typical results are presented for these experiments in terms of:
- Chromium recovery.
- Distribution of principal alloying elements; separation between chromium and iron; separation between chromium and cobalt and nickel; and separation between chromium and other alloying agents, such as titanium, tungsten and tantalum.
- Oxygen utilization.
Chromium Recovery
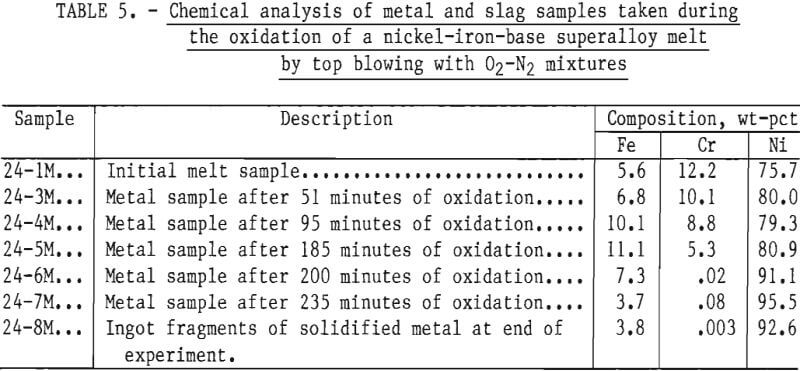
Experimental results showed that given sufficient oxygen the chromium content of a molten bath of superalloy scrap could be reduced almost quantitatively to zero. Indicative of this are the results obtained by top blowing oxygen-nitrogen mixtures onto the surface of a molten bath of B-1900 and Inconel 600 superalloy scrap initially containing 12.2 pct chromium. In this experiment, the chromium content of the metal phase was reduced from 12.2 to 0.003 pct in a period of about 3 hours, using gaseous mixtures consisting of either 50 pct oxygen and 50 pct nitrogen (by volume) or pure oxygen. The bath temperature ranged from 1,530° to 1,700° C. An overall material balance for this particular experiment is given in table 4. Chemical analyses of metal samples obtained during the run are given in table 5, and the chromium to nickel ratio in metal samples as a function of oxidation time is given in figure 9. Also shown in figure 9 are injection rates and gas composition for oxidizing gases.

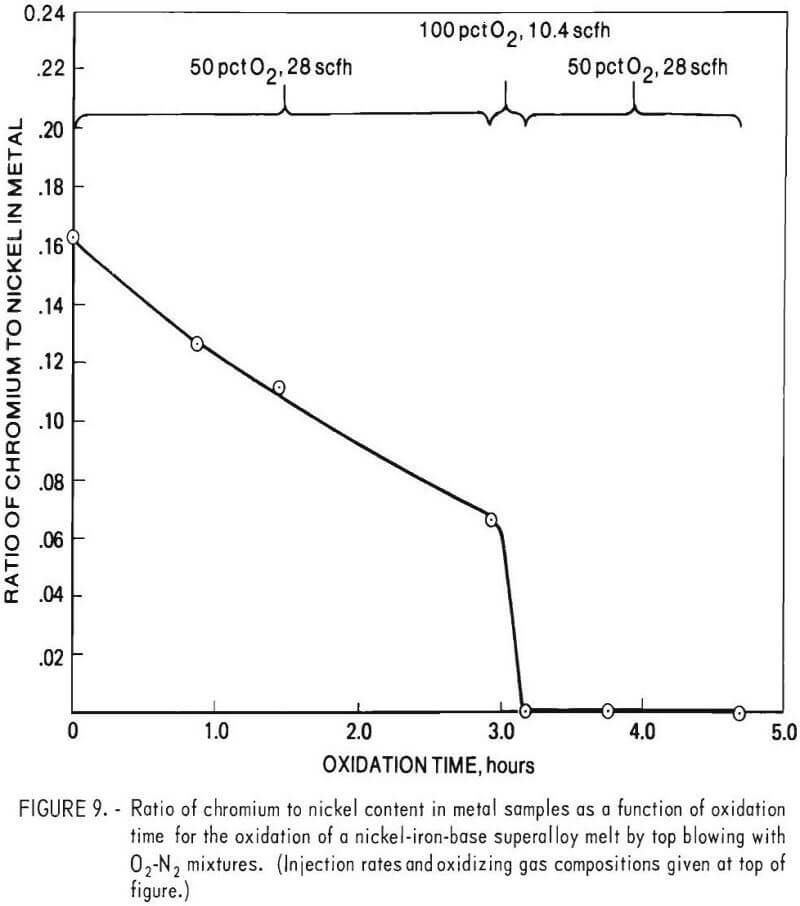
A material balance for this experiment, shown in table 6, was calculated based on:
- Initial weight of superalloy scrap charged to the crucible.
- Chemical analysis before oxidation of molten metal (Sample 24-1M) for iron, chromium, nickel, cobalt, and “other” elements calculated by difference.
- Final chemical analysis (at the end of the oxidation experiment) of metal phase (Sample 24-8M) for the elements listed.
The approach used in constructing the material balance assumed that nickel and cobalt remained in the melt phase (that is, were not oxidized) and that chromium, iron, and “other” species were partially removed by oxidation. The oxygen content of the slag was estimated by assuming that chromium oxidized to Cr2O3, iron to FeO, and “others” to M0O2, TaO2, Al2O3, and TiO2. The “other” category was partitioned into molybdenum, tantalum, aluminum, and titanium based upon the nominal compositions of Inconel 600 and B-1900 superalloys as given by INCO.
The material balance, given in table 6, shows that the computed output of 3,905 grams compares well with the experimental weight of 3,997 grams. (The experimental weight was known to be higher than the true value because of difficulties in removing some alumina particles from the metal ingot.) In addition, the calculated concentrations of elements in the metal phase compare well with measured values, as shown in table 7.
Based on the results of the material balance given in table 6, the chromium removal for this oxidation experiment was greater than 99.9 pct.
To illustrate high chromium removal with different metal composition, a charge consisting of about equal weights (6,526 grams total) of AISI 430 stainless steel and carbon steel was top-blown with a 50 pct oxygen-50 pct nitrogen gas mixture. Lime-silica flux was added at 1.7 pct by weight of metal charge. Metal samples were taken periodically during the run. Compositions are given in table 8. Readings taken with a platinum/platinum-rhodium thermocouple placed above the bath, indicated that the temperature of the bath ranged from 1,300° to 1,420° C with an average of about 1,375° C. These temperatures indicate an average bath temperature of approximately 1,525° C, slightly lower than normally used in oxidation experiments.
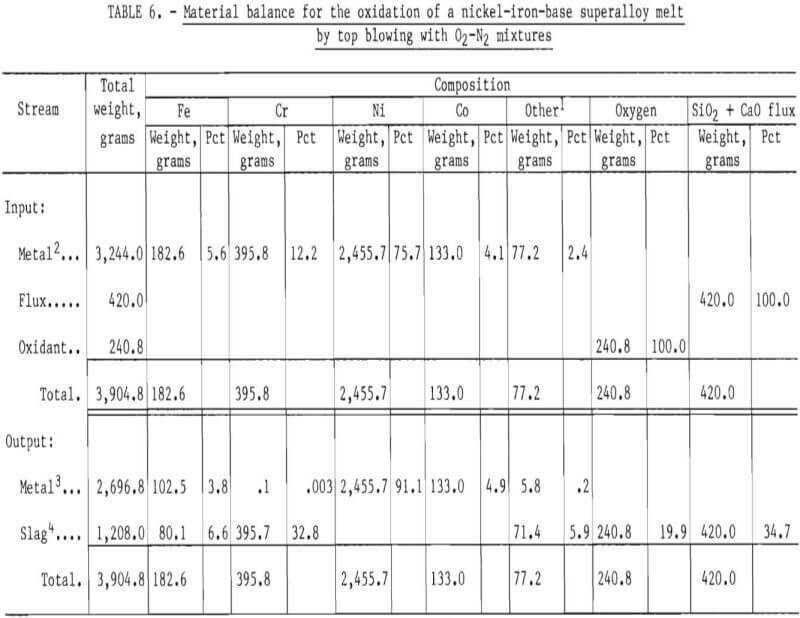
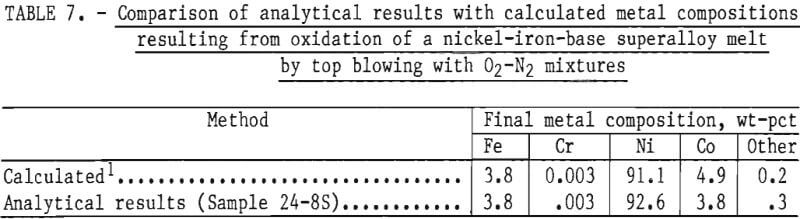
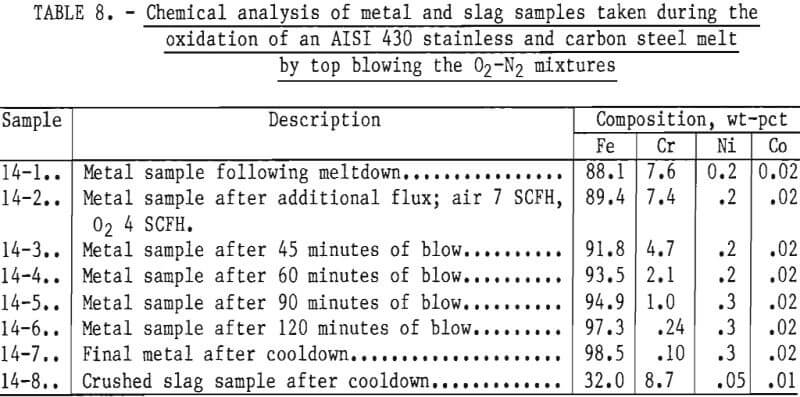
Figure 10 presents a plot of chromium content of the bath as a function of oxidation time. During this run, the chromium content of the metal was reduced from 7.6 to 0.11 pct. Based on the assumption that nickel and cobalt were not oxidized (justifiable from slag analyses), a final metal weight of 4,487 grams was estimated. The chromium removal was therefore 99.1 pct.
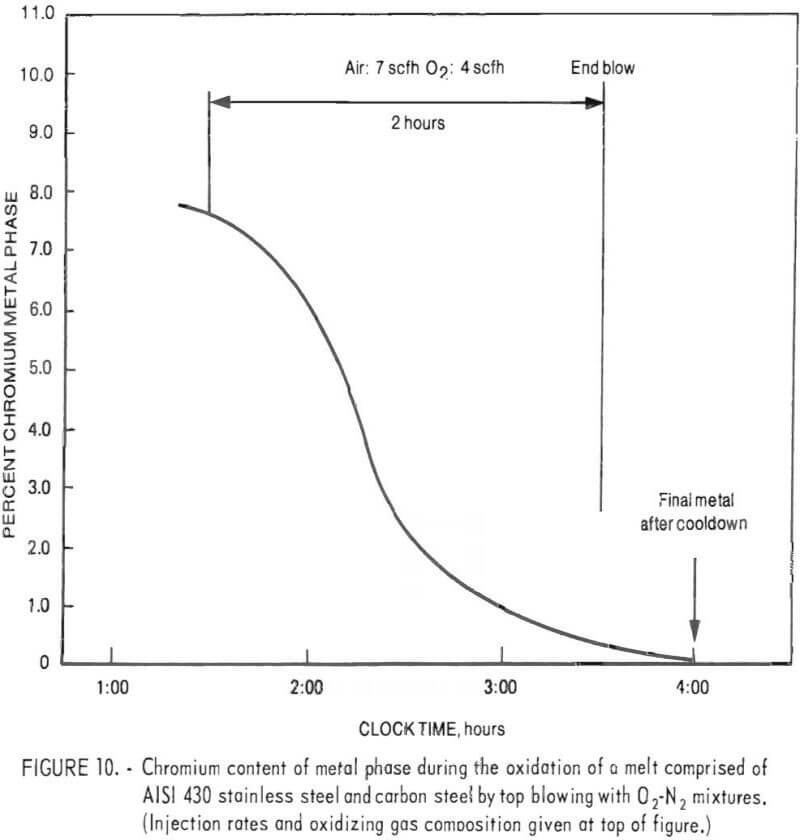
In an example of an experiment illustrating the use of a gaseous oxidant starting with AISI 430 stainless steel and carbon steel, the chromium content of the metal phase was reduced from 3.1 to 0.3 pct in 80 minutes using a top-blown mixture of 50 pct oxygen and 50 pct nitrogen. The overall material balance and chemical analyses of samples for this experiment are given in tables 9 and 10, respectively. Temperatures within the crucible averaged 1,660° C during the run. Based on the estimated weight of metal in the crucible at the end of the experiment, and final chemical analyses, chromium removal was 92.1 pct.
From thermodynamic considerations, any nickel inadvertently oxidized should be capable of oxidizing residual chromium in the metal phase (fig. 1) by the following reaction:
3NiO + 2Cr → Cr2O3 + 3Ni
To demonstrate that this would occur, a charge consisting of 1,230 grams of AISI 430 stainless steel analyzing 14.4 pct chromium and 493 grams of nickel oxide was melted and allowed to equilibrate for an hour in an inert atmosphere. The amount of nickel oxide used was in excess of that required for chromium oxidation. After equilibration the power was turned off and, following cool-down, weights of metal and oxide phases were estimated and samples of metal and oxide analyzed. A total material balance is shown in table 11. The compositions of the metal phase before and after equilibration and the oxide phase after equilibration are shown in table 12. As indicated, the chromium content of the metal phase was reduced from 14.4 to 0.1 pct. Based on the weights of metal and composition of the metal phase before and after equilibration, chromium removal was estimated to be 99.2 pct.
Distribution of Principal Elements
Table 14 summarizes the distribution of principal alloying elements in oxidation experiments. These results were calculated from analytical results of metal samples prior to oxidation and the computed composition of the metal phase after oxidation, as specified by material balance calculations (for example, see table 6). The separation between chromium and the principal alloying elements is discussed.
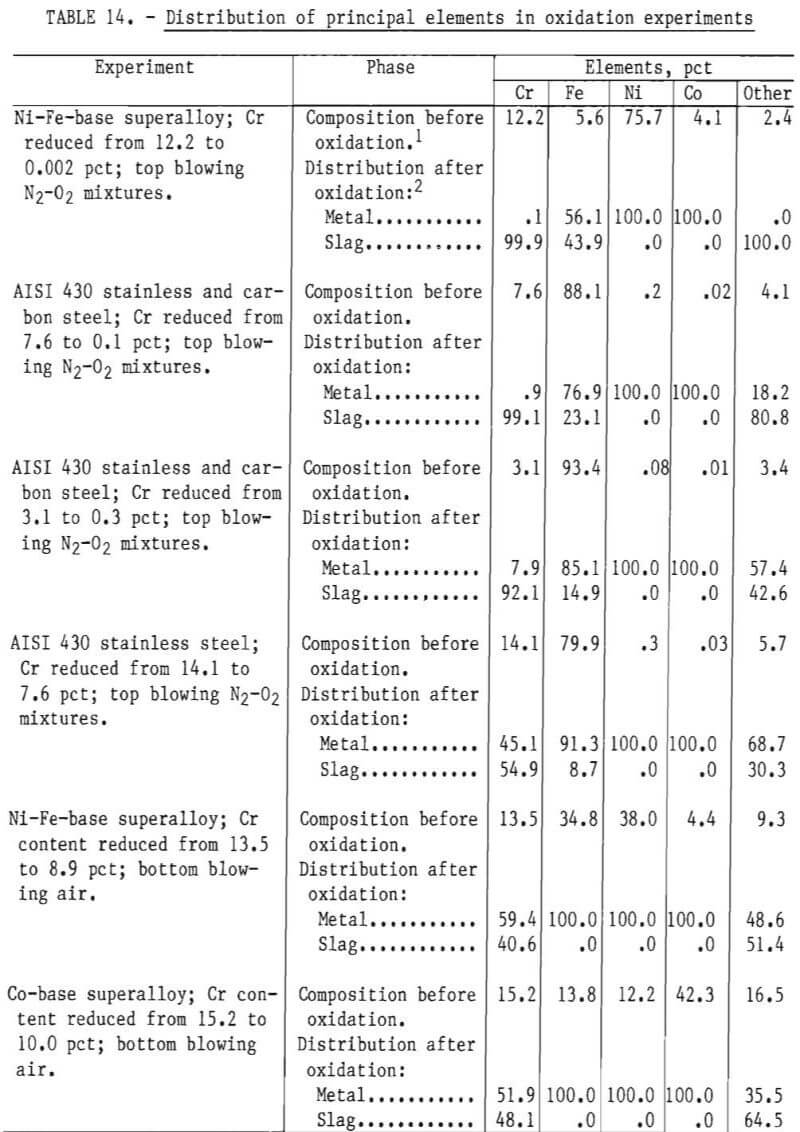
Separation Between Chromium and Iron
An examination of the data in table 14 indicates that:
- A significant amount of iron was oxidized into the slag phase during top blowing.
- Even with significant quantities of iron oxidation occurring with top blowing, slags were obtained with Cr-Fe ratios exceeding 1.0.
- Comparison of top-blowing and bottom-blowing experiments for melts having similar levels of chromium indicate that with bottom blowing chromium can be oxidized with little or no iron oxidation.
In the induction furnace tests having cold and rather viscous slags, gas-metal contact with top blowing appeared to be poor. Gas-metal contact was excellent with bottom blowing. Thus, bottom blowing offers an interesting alternative for achieving chromium oxidation while preventing iron oxidation.
Separation Between Chromium and Cobalt and Nickel
Analysis of the slag phase from various oxidation experiments indicated low levels of nickel and cobalt. Material balance calculations, assuming all nickel and cobalt stayed in the metal phase, gave weights of slag and metal phase that compared well with experimentally determined weights. Analytical data thus confirm theoretical free energy considerations (see fig. 1) that nickel and cobalt remain in the residual metal phase during chromium oxidation.
Separation Between Chromium and Other Alloying Elements
In the experimental program the total amount of other alloying elements (such as titanium, tungsten, and tantalum) was analyzed by difference. It is seen from table 14 that these elements exhibit a tendency to concentrate in the slag phase. This result was to be expected as many of the elements are more oxidizable than chromium, that is, the Ellingham diagram (fig. 1) indicates that these elements have more negative free energy of oxide formation than does chromium.
It must be noted that actual concentration of minor alloying elements in the metal and slag phases was not determined in this investigation. Although the oxidation and subsequent reduction of chromium was studied in the presence of these minor alloying elements, the determination of the distribution of these elements between slag and metal phases during oxidation and reduction was deemed outside of the scope of this project. Since the purity of ferro-chromium product is dependent on the extent to which these elements are present, report to the slag during oxidation, and are then reduced into the ferrochromium during reduction, it is important that this issue be studied in later pilot-plant investigations when specific types of scrap available to this process become more clearly delineated.
Oxygen Utilization
During oxidation experiments, a polarographic-type oxygen sensor was used online to measure the oxygen content of off-gases. Since pressure measurements made in the system indicated a positive gage pressure, oxygen sensor measurements were interpreted as being representative of off-gases leaving the crucible.
In top-blowing experiments with oxygen-nitrogen mixtures, oxygen utilization was a function of the amount of slag in the system. Typically with very little slag, oxygen utilization would be high (about 100 pct) and drop off to low values (less than 20 pct) as slag crusting occurred. Physically breaking the slag crust would cause oxygen utilization to increase because fresh metal surface was exposed to the oxidizing gas.
In bottom-blowing experiments with air, oxygen utilization was high, in excess of 95 pct.
Slag-Metal Separation
Several experiments were performed in which attempts were made to remove chromium-bearing slags from the crucible (in the 50-kW furnace) while retaining molten metal, that is, to perform a high-temperature separation of slag and metal phases. Since the induction furnace was not equipped with a slag gate for pouring, attempts were made with various kinds of scoops. In general, these attempts were unsuccessful—at best, less than 20 pct of the slag could be removed with the scoop. However, there were some indications that silica to lime ratio higher than 2:1 (as used in most oxidation fluxes) improved slag fluidity and by extension the ease by which slags could be removed. The optimal acid-base ratio for slag fluidity could not be determined from these tests.
Reduction of Chromium-Bearing Slags
The purpose of the reduction experiments in the experimental program was to demonstrate that chromium could be reduced from slags obtained by the oxidation of superalloy melts. Accordingly, raw materials were chosen to simulate materials that would be obtained in pilot-plant testing. In the oxidation experiments of the experimental program, slags containing 20 to 30 pct chromium and Cr-Fe ratios in excess of 1.7 were obtained, as shown in table 15. These slags are similar in composition to slags obtained in a pilot-scale test to produce ferrochromium from iron-rich chromites. For example, as described in reference 3, a lime-chromite slag containing 20 pct chromium was produced using iron-rich chromite with a Cr-Fe ratio of 1.6. This slag was reduced with ferrosilicon (75 pct Si) to produce ferrochromium with a chromium yield exceeding 85 pct. For chromites with higher Cr-Fe ratios, the reduction step has been demonstrated by industrial ferroalloy practice.
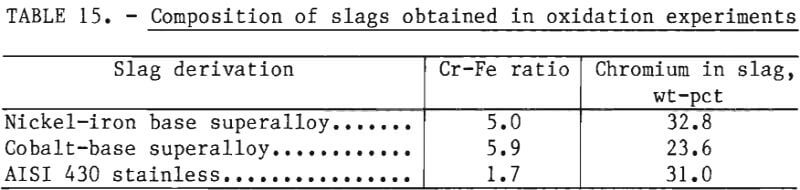
Reduction with Ferrosilicon
In the experimental program, the reduction of synthetic chromium-bearing slags with ferrosilicon was carried out in the 20-kW induction furnace in 2-inch alumina crucibles. These experiments showed that chromium can be reduced from synthetic slags containing as little as 10.3 pct chromium with ferrosilicon to produce a product rich in chromium (65.3 pct Cr) at high chromium recoveries exceeding 95 pct.
Further confirmation was obtained of the ability to reduce the chromium from slags produced by oxidation of superalloy and stainless steel melts using the 50-kW induction furnace. The experiments consisted of addition of ferrosilicon (75 pct Si) to a molten bath of chromium-bearing slag covering residual metal from an oxidation experiment. As the reduction proceeded, the chromium content of the residual metal phase increased. The reduction was followed by means of metal samples taken for analysis during the experiment.
For example, slag obtained from the oxidation of an iron-nickel-base superalloy was reduced with 75 pct ferrosilicon to recover over 95 pct of the chromium from the slag. At the start of the experiment the slag analyzed 30 pct Cr and the residual metal phase analyzed 8.9 pct Cr. The bath temperature was maintained between 1,525° and 1,575° C during the experiment.
In order to reduce the chromium from the slag phase, 200 grams of ferrosilicon were initially added to the bath. After 40 minutes of equilibration, another 50 grams of Fe-Si was added. Samples of metal phase were taken during the experiment and a slag sample towards the end of the experiment. An overall input-output balance for this experiment is shown in table 16. Chemical analyses of metal and slag samples obtained during the experiment are shown in table 17.
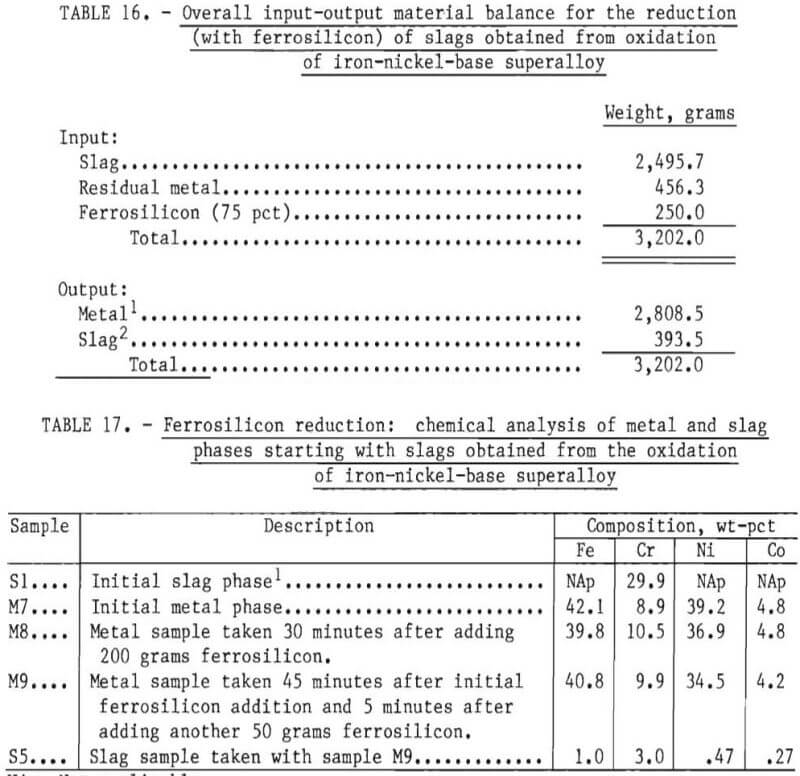
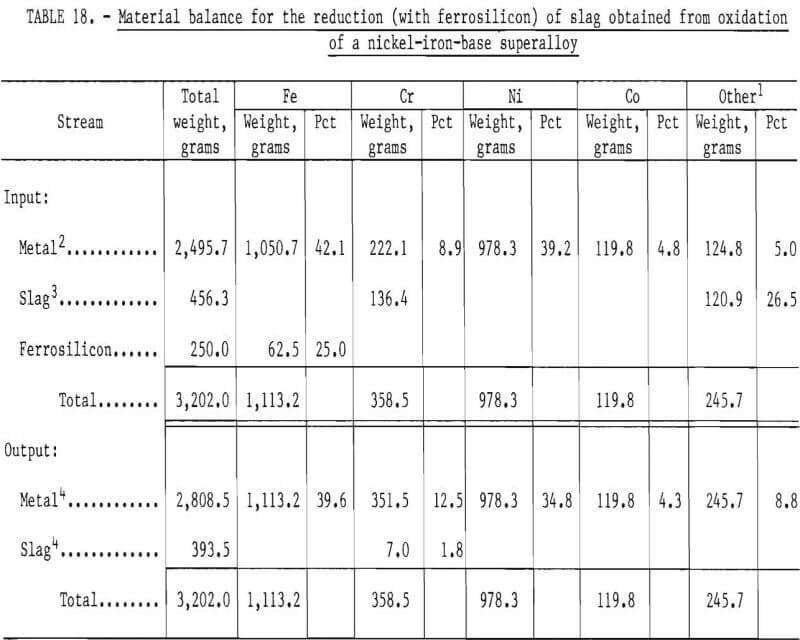
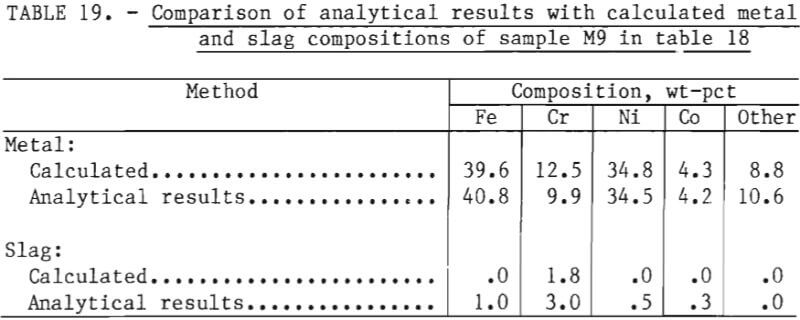
A material balance for this experiment, shown in table 18, was calculated based on estimated weights of slag and metal initially present in the crucible, the composition of metal as given by sample M7, and an estimated slag composition SI. It was assumed that chromium and “other” metallic elements were reduced from the slag phase into the metal phase by reaction with free silicon present in ferrosilicon. Since silicon analyses were not performed, the efficiency of silicon use could not be investigated. As shown in table 19, the final compositions of the slag and metal phase agree well with measured compositions. The chromium recovery based on the material balance was 95 pct.
Reduction With Other Reducing Agents
Preliminary test work was done with aluminum and carbon as reducing agents on slags obtained from oxidation experiments of cobalt-based superalloy. Results indicate that, as expected, aluminum and carbon can be used as reducing agents for chromium oxide.
Future Research
Although the technical feasibility to recovery chromium from critical scrap metals has been established in small-scale induction furnace experiments, two technical procedures which were difficult to demonstrate are suggested for future research.
- To assure good oxygen-metal contact during chromium oxidation, it is proposed that future pilot-plant experiments be conducted in an electric arc furnace to insure a high-temperature slag to promote fluidity. Oxygen lancing onto the top surface of the melt is proposed as the simplest method of achieving chromium oxidation. If this should prove to be inefficient due to crust formation in the slag phase, submerged air injection into the molten metal bath is recommended either by a consumable lance or by introduction through the side walls of the furnace as has been practiced in steelmaking (as, for example, at Sidney Steel in Nova Scotia or Armco Steel in Houston, Tex.).
- The amount of air to be introduced to oxidize chromium depends on initial scrap metal composition and degree of oxygen utilization. It is proposed that an oxygen analyzer (for the off-gases) and an X-ray analyzer (for the metal phase samples) be used to determine when the chromium has been oxidized out of the metal phase.
In addition to these two technical procedures, it is also proposed that other features of the process that were outside of the scope of the research program be studied in detail, preferably on a pilot-plant scale. They include:
- The separation between chromium and minor alloying agents in superalloy scrap such as tungsten, molybdenum, and tantalum. Data obtained in this investigation indicate that these alloying elements tend to report to the slag phase during oxidation. Although appropriate Cr-Fe ratios have been indicated for final ferrochromium product in these experiments, the purity of ferrochromium with respect to other elements has not. Hence, it is important that detailed chemical analysis of intermediate and final products (that is, accounting for specific alloying elements such as Mo, Ta, W, Al) be carried out so that the distribution of these elements in intermediate and final products, particularly ferrochromium, can be determined. This approach should also include elements associated with reductants, such as ferrosilicon, and possibly some of the fluxing reagents.
- To reduce chromium oxidation periods, the relatively slow oxygen injection rates employed in small scale experiments should be significantly increased on a pilot-plant scale, if feasible.
- Reduction of chromium-bearing slags with ferrosilicon required approximately 40 minutes to achieve equilibrium. Tests should be conducted with fluxes having silica to lime ratios exceeding 2:1. This should improve slag fluidity and possibly result in more effective ferrosilicon-slag contact that would decrease time requirements. Other approaches to obtain better ferrosilicon-slag contact would be the use of slag additives and agitation. Related studies on commercial slags are in progress at the Bureau of Mines Rolla (Missouri) Research Center using laboratory-size arc furnace melts. Initial efforts are aimed at establishing higher distribution ratios of chromium between metal and slag using silicon and carbon reductants. Research is also being directed towards recovery of Cr, Co, Ni, and other critical metals from alloy scrap using electrolytic techniques.
- The electric arc furnace for larger scale studies can be either an open-arc (direct-arc) or a submerged-arc. However, a report by EPA indicates that submerged-arc furnace operations produce a larger evolution of particulate matter compared to the open-arc. This suggests that parallel studies might be worthwhile to establish the optimum mode of operation, that is, submerged-arc or open-arc.
- Other issues, such as degree of slag-metal separation that can be achieved, and refractory consumption, are clearly important but are judged to be less critical in that they have been demonstrated in past work and in prior commercial practice.
Based on the experimental studies described in this report, descriptive material and cost estimates were developed for a pilot-plant operation to process at least 100 pounds per hour of scrap. Details of the pilot-plant design have been described in Open-File Report No. 75-80. This report can be inspected at the Library, U.S. Department of the Interior, Washington, D.C., or the Bureau of Mines facilities in Albany, Oreg., Avondale, Md., Denver, Colo., Pittsburgh, Pa., Reno, Nev., Rolla, Mo., Salt Lake City, Utah, Spokane Wash., Tuscaloosa, Ala., and Twin Cities, Minn.
Conclusions
Data obtained from the experimental program indicated that:
- It was possible to oxidize and remove chromium from a variety of chromium-bearing scrap types regardless of the other alloying constituents present. Chromium removals exceeding 99 pct could be achieved. There was no evidence of nickel or cobalt oxidation while chromium oxidized. The results are consistent with theoretical thermodynamic principles used to devise the conceptual flowsheet for the process.
- As expected from commercial practice, no difficulty was experienced in reducing chromium oxide (in a slag phase) to chromium metal. It was possible using ferrosilicon to recover up to 95 pct of the chromium initially present in a slag phase.
- It was possible to obtain sufficiently good iron-chromium separation to produce ferrochromium product suitable for steelmaking. Using synthetic slags (that is, slags that were not produced in an oxidation experiment), a ferrochromium product was produced that contained 65 pct chromium.
Appendix.—Analytical Methods
The category of superalloys includes numerous alloy classifications. The variety and complexity of these combinations requires a more complex methodology for chemical analysis than would be necessary if only a limited number of elements were found in a given sample. A careful choice must be made of analytical method and sample preparation technique to assure that accuracy and precision are obtained for the key elements to be analyzed in samples which may vary widely in chemical composition.
The analytical work undertaken in support of the experimental program involved the determination of cobalt, chromium, iron, and nickel in superalloy scrap starting materials, intermediate reaction products and slags, and final product metals and slags. No single analytical procedure applicable to this variety of sample types and composition was available in the chemical literature. Thus it was necessary at the beginning of the project to develop an appropriate analytical procedure. This effort included a brief review of standard analytical methods ordinarily employed for similar analyses, selection of an appropriate analytical method, development of a suitable sample preparation procedure, and analysis of standard reference materials for the purpose of validating the resulting analytical methodology. Each of these steps is briefly described.
Standard Analytical Methods
Emission spectroscopy and X-ray spectroscopy have generally been the analytical methods of choice in the industry because of their speed and resulting economy when large numbers of analyses of a single alloy type are to be made.
Emission spectroscopic procedures are applicable to the determination of all metallic constituents in ferrous alloys and are especially valuable for elemental concentrations below 0.1 pct. Various forms of solid samples or dissolved samples may be analyzed. Limitations include the need for careful standardization of procedures, the need for different calibration curves for each type of sample matrix, and the difficulty in determining high concentrations with accuracy and precision. Instruments employing plasma excitation sources have recently become commercially available and have reduced or eliminated many of these former disadvantages.
X-ray spectroscopy can be used for determining all but the lighter elements in ferrous alloys. It is better suited than older emission spectroscopic techniques to determine elements present in high concentrations. Volumetric, gravimetric, and colorimetric methods have long been associated with the analysis of certain metallurgical materials when multiple determinations of a single element are required. Prior separation to eliminate interferences due to the presence of one or more additional elements in the sample must usually be effected when these methods are used. The accuracy, precision, and selectivity obtainable with atomic absorption (AA) spectrometry are considered to be competitive in many cases with classical techniques, with the result that this method has found increasing use in industry. A limitation of AA methods is that sample solutions containing high elemental concentrations may need to be diluted several-fold to bring them into a concentration range appropriate for analysis.
Selection of Analytical Method
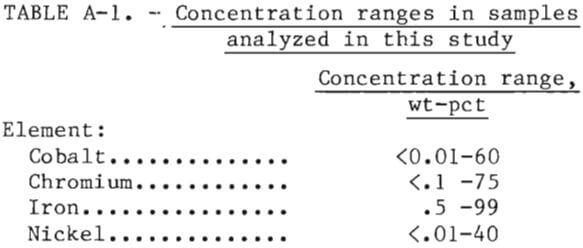
The analytical procedure selected should be capable of handling the anticipated concentrations range of Co, Cr, Fe, and Ni as shown in table A-1. Of the analytical methods described, plasma emission spectroscopy was identified as having the best combination of applicability to these concentration ranges, accuracy, precision, speed, and flexibility with respect to choice of elements for analysis. Thus, a plasma emission spectrometer equipped with a dc argon plasma excitation source was used for all the analytical work described in this report. Close matching of sample and standard matrices is much less critical than in other emission spectroscopic techniques, calibration is less extensive and is required less frequently, and the sample throughput is higher. A limitation of this technique is that samples must be in solution for presentation to the instrument. This necessitated an effort at the beginning of the project to develop an appropriate sample preparation procedure.
Development of Sample Preparation Procedure
Most ferrous alloys are dissolved by the common inorganic acids (HCl, HNO3, H2SO4), singly or in combination. However, the sampling technique used in this study to obtain samples of intermediate reaction products was expected to result in the possible presence of difficult soluble silicates in those samples. Slag samples were also expected to be resistant to acid attack. The need to assure complete dissolution of samples containing both free metals and silicates suggested that the use of a two-step sample preparation procedure including preliminary acid dissolution followed by fusion of insoluble matter was apropriate.
The acid dissolution procedure described in ASTM Method E 484-73, “Standard Method for Optical Emission Spectrometric Analysis by the Solution-Reservoir Cup Technique,” had been shown in previous work to provide complete dissolution of samples of structural steels. Preliminary qualitative tests on samples of superalloy starting materials indicated that this procedure gave clear solutions with no visible residue. Slag samples, however, were not completely dissolved by this acid treatment, so the classical sodium-carbonate fusion approach for difficult soluble silicates was evaluated for dissolution of insoluble residue. This fusion approach was found to completely dissolve slag samples if the flux and sample mixture were heated for a minimum of 2 hours. The acid and fusion steps were combined with plasma emission spectroscopic analysis in the method described in table A-2. This method was used for the analysis of all samples.
Analysis of Standard Reference Materials
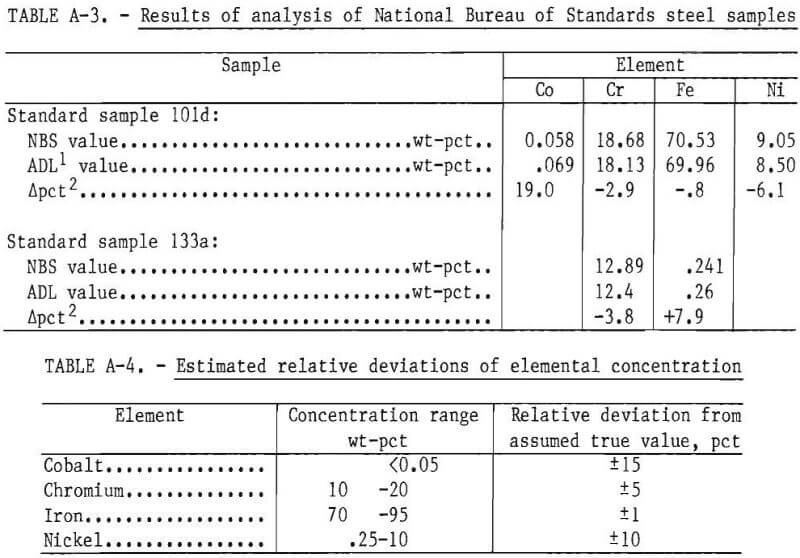
Two National Bureau of Standards (NBS) steel samples were obtained and analyzed using the analytical procedure described in table A-2. The results are shown in table A-3. These results indicated that the relative standard deviation of the determined concentration for a given element could be expected to be within the indicated percentages of the assumed true value as given in table A-4.

Sample preparation of spectroscopic analysis
| Step | Description |
| 1. | Weight out 0.1 gram of sample into 50-ml beaker. |
| 2. | Add 1 ml of 1:1 nitric acid and cover with a watch glass. |
| 3. | When the initial vigorous reaction ceases, add 4 ml of 1:2 hydrochloric acid.
For metal samples of the type used, 5 to 10 minutes of gentle heating should be sufficient to completely dissolve the sample. Cool and dilute the resulting mixture to approximately 25 ml. Filter through Whatman No. 41 filter paper or equivalent. Quantitatively transfer the filtrate to a 50-ml volumetric flask. Fuse the residue in a platinum crucible using 5 grams of sodium carbonate as a flux. The sample should be kept in quiet fusion for 2 hours. Cool and dissolve the cake using 75 ml of 1:2 hydrochloric acid. Quantitatively transfer the resulting solution to a 100-ml volumetric flask. |
| 4. | Determine the content of Fe, Cr, Ni, and Co in the sample solutions by plasma emission spectroscopy. Calibrate the instrument according to the manufacturer’s directions using calibration standards prepared by serial dilution of 1,000-ppm aqueous standard solutions. Each standard should contain all four elements at appropriate concentrations. Standards for analysis of steel samples and the acid filtrate portion of slag samples should also contain 1 ml of 1:1 nitric acid and 4 ml of 1:2 hydrochloric acid. Standards for analysis of the fused portion of slag samples should contain 5 grams of sodium carbonate to which 75 ml of 1:2 hydrochloric acid have been added. |
| Emission lines for the elements are: | |
| Analytical lines wavelength, A | |
| Element: | |
| Fe | 2,404.88 |
| Cr | 2,835.63 |
| Ni | 2,270.21 |
| Co | 3,453.51 |
Note.—Final results should be expressed as percentages of the sample weight taken for analysis.
Summary of Analytical Procedure
The analytical procedure developed for this study included acid pre-treatment and fusion steps for sample dissolution followed by analysis by plasma emission spectrometry. The procedure was applicable to the ranges of sample types and compositions encountered. Detection limits estimated from the signal to noise ratios for actual sample solutions were on the order of 0.01 wt-pct of the respective elements in metals and slags.
