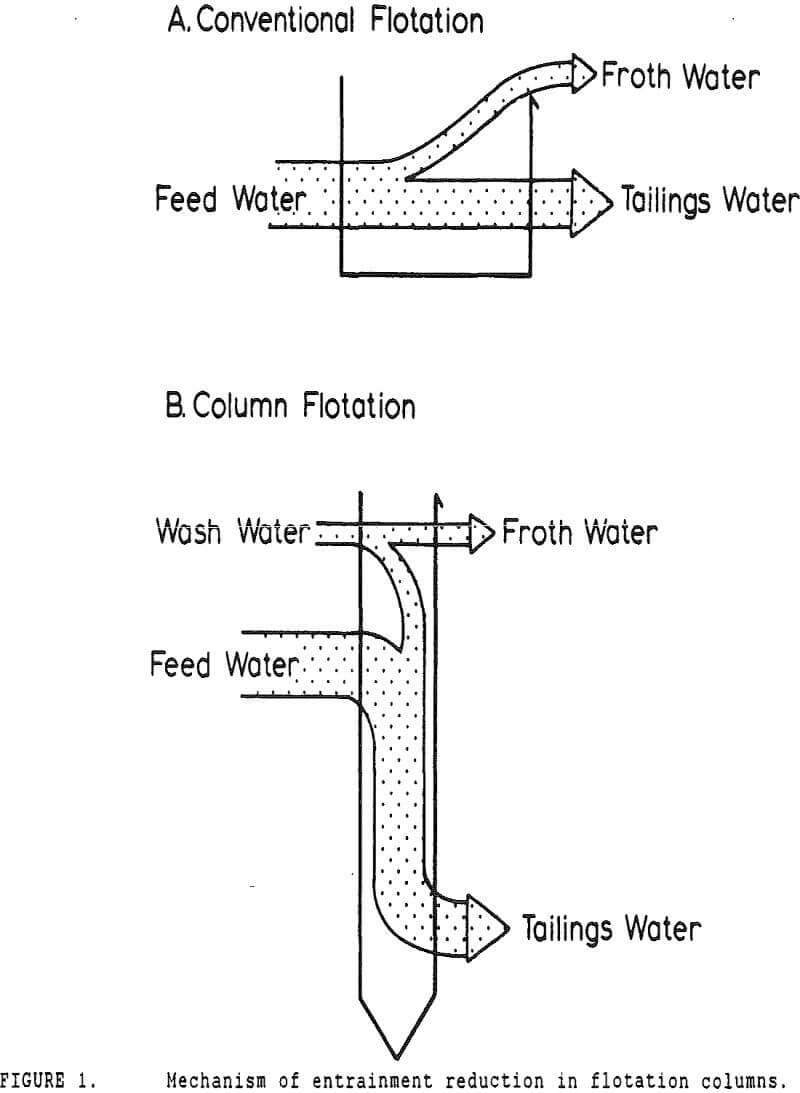
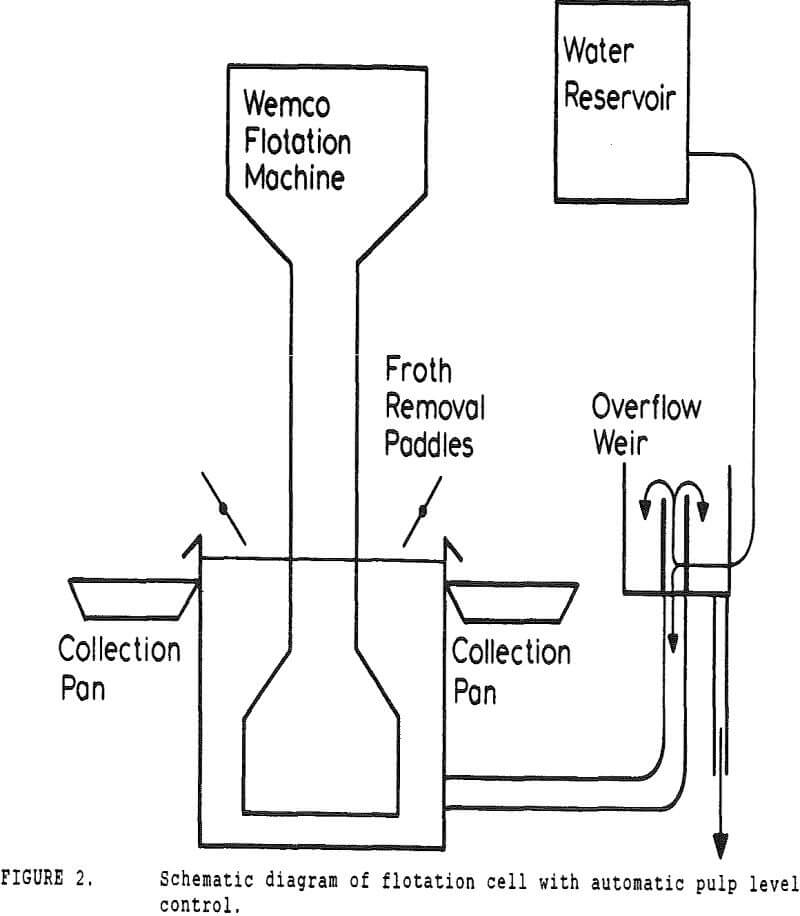
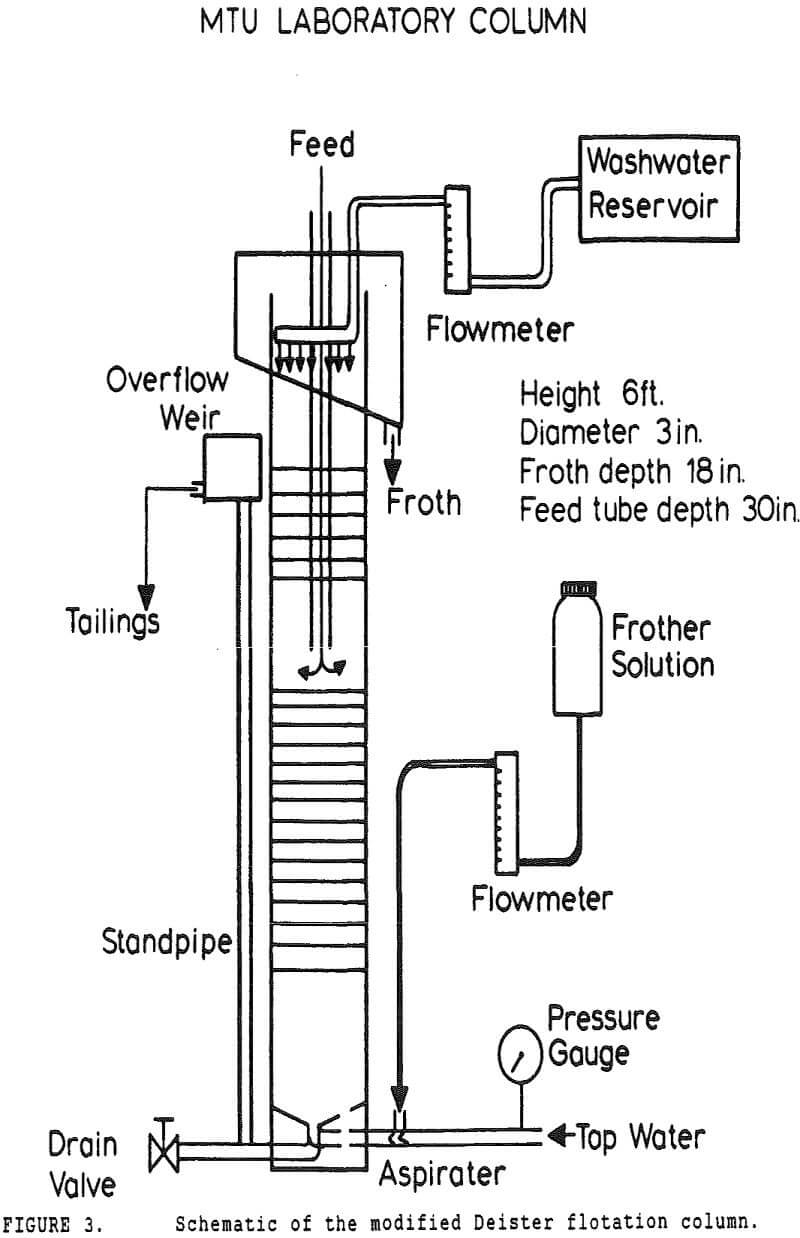
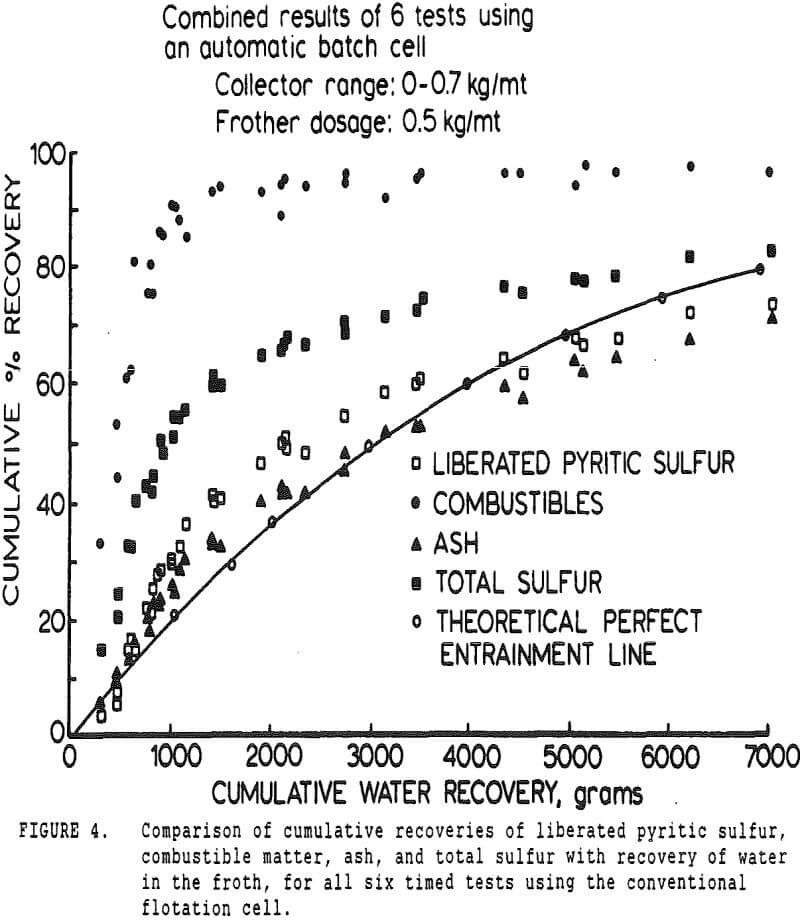
In many coal flotation operations, a significant amount of apparently liberated pyrite is seen to report to the froth, thus raising the sulfur content of the clean product and reducing its economic value. Prevention of this unwanted recovery of pyrite is therefore desirable.
Theoretical Discussion
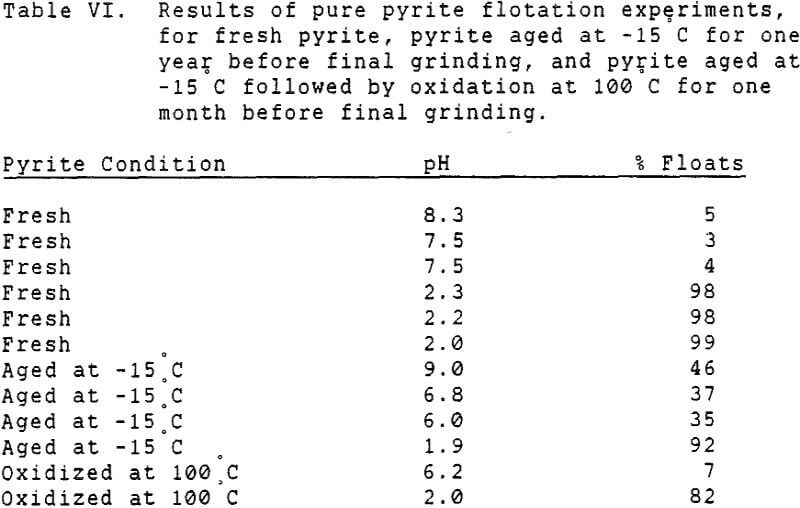
It has long been known that mineral pyrite has some hydrophobic tendency, as do several other sulfide minerals. However, this tendency is normally very slight, and significant flotation of pyrite requires addition of a collector such as a xanthate. The natural floatability of pyrite is also strongly pH dependent, with the highest floatability in acidic solutions.
Many investigators have attempted to use various chemicals as depressants for coal pyrite in order to reduce the sulfur content of the product, with some success, However, in most reported experiments it has not been clear whether the decrease in pyrite flotation was due to reduced pyrite hydrophobicity or to other factors such as improved froth drainage, as the quantity of entrained water in the froth is typically not measured. Also, since pyrite depressants reduce the floatability of coal slightly, it is reasonable to expect that fewer locked particles will float in the presence of these reagents, and that the quantity of pyrite floating can be reduced by this means as well.
Experimental Procedures and Results
In order to determine whether the pyrite in a particular Pittsburgh Seam coal was floating by locking/entrainment or by true flotation, two sets of experiments were carried out.
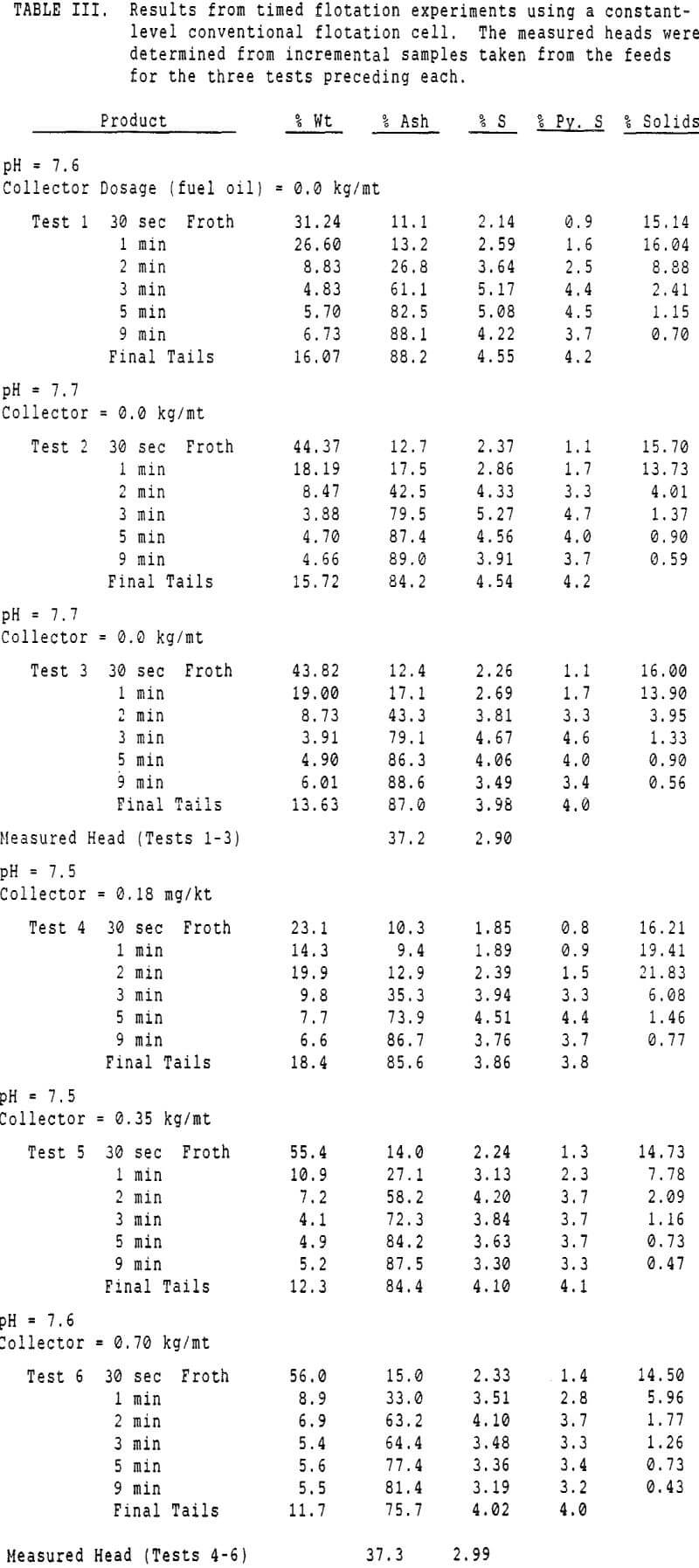
The first objective was to attempt to estimate the relative proportions of sulfur reporting to a coal froth by entrainment and true flotation. To accomplish this, it was necessary to measure the recovery of coal, ash, water, and the various forms of sulfur as a function of time. Variations in collector dosage were intended to vary the coal flotation rate, and determine whether pyrite flotation was or was not sensitive to collector dosage.
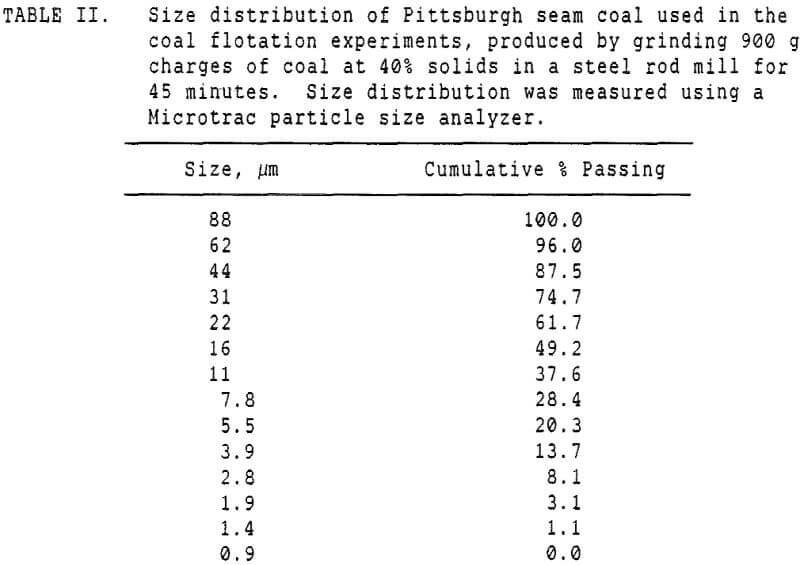
These experiments used 250 gram charges of a Pittsburgh seam coal which had been stored at -20 C prior to use, to retard oxidation. The freshly ground coal was then divided into 250 gm charges for flotation, and floated as soon thereafter as possible.
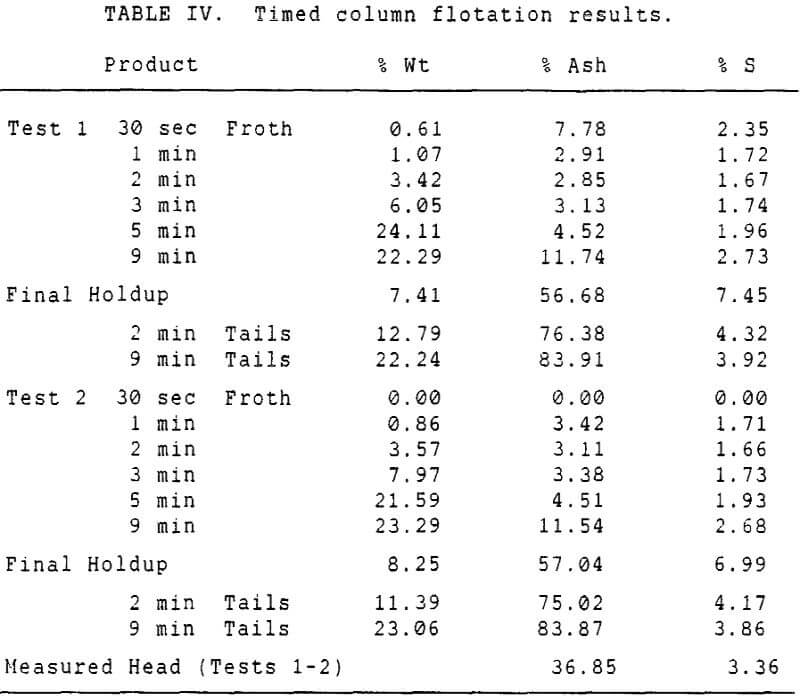
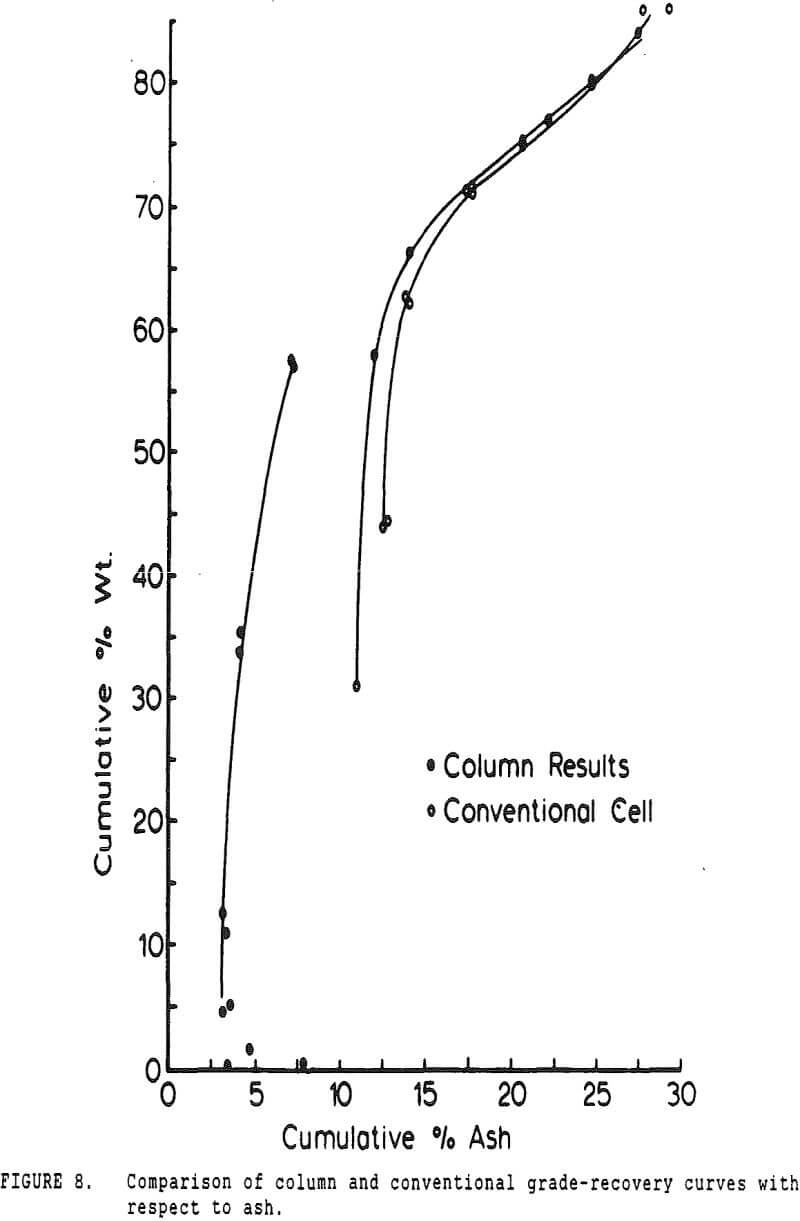
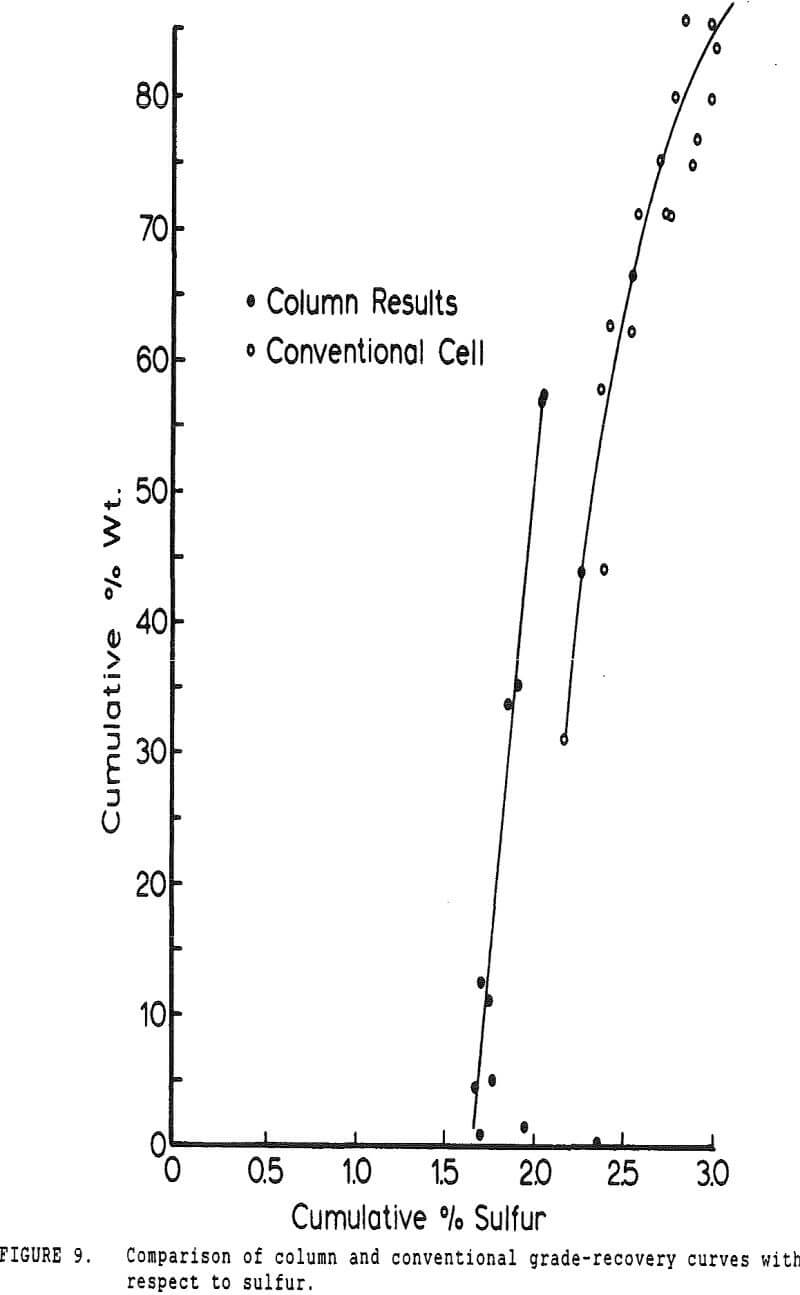
Experiments with column flotation were intended to determine the behavior of the coal components when entrainment was minimized by froth washing. Flotation experiments were carried out in a laboratory-scale coal flotation column, using coal prepared in the same manner as that used in the conventional coal flotation tests.
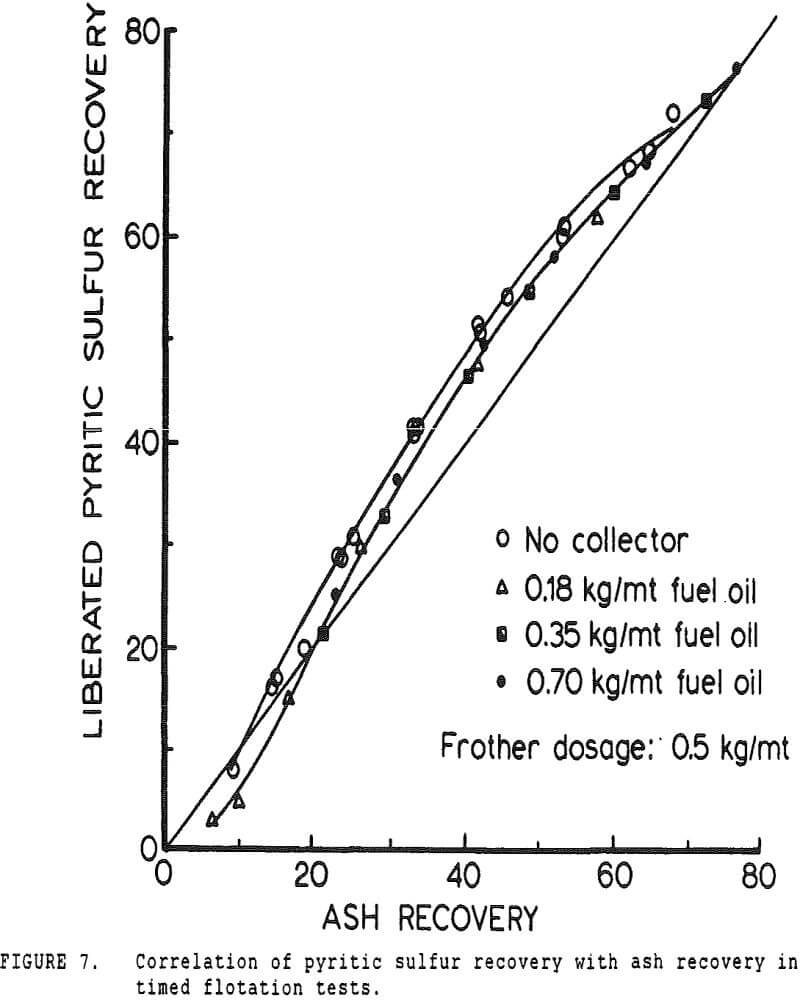
Since it is not practical to liberate coal pyrite completely from the locked coal particles, experiments were conducted using mineral pyrite to determine the extent to which it was floatable using only fuel oil as a collector. Although mineral pyrite does not have the same surface chemical properties as coal pyrite, it is nevertheless the only available model which can be obtained readily.
Discussion
Determining conclusively whether pyrite is being recovered by true flotation, locking of particles, or entrainment is technologically impractical at this time. Approximate estimations can be achieved by correlating the recovery of a given component (coal, ash, etc.) with the recovery of water in the froth. For these data to be meaningful, it is first necessary to develop a method of accounting for each type of sulfur present in coal. Sulfur is predominantly in the form of organic sulfur, liberated pyritic sulfur, and pyritic sulfur intermixed with coal.
Standard analysis techniques allow for quantitative measurement of total sulfur and pyritic sulfur, but no practical methods are available for differentiating between liberated and unliberated pyritic sulfur for large numbers of particles.
- The liberated pyrite is always less floatable than the most floatable coal particles when no collector is added, and therefore the fastest-floating coal in the absence of collector will be free of liberated pyrite if entrainment is minimized.
- The locked pyrite and organic sulfur are uniformly distributed throughout the combustible fraction of the coal.
This value of 1.7% locked and organic sulfur is further supported by a heavy-liquid separation of the feed, at a specific gravity of 1.35. The low-gravity float product was found to have a total sulfur content of 1.79 wt.%, at a recovery of 20% wt.
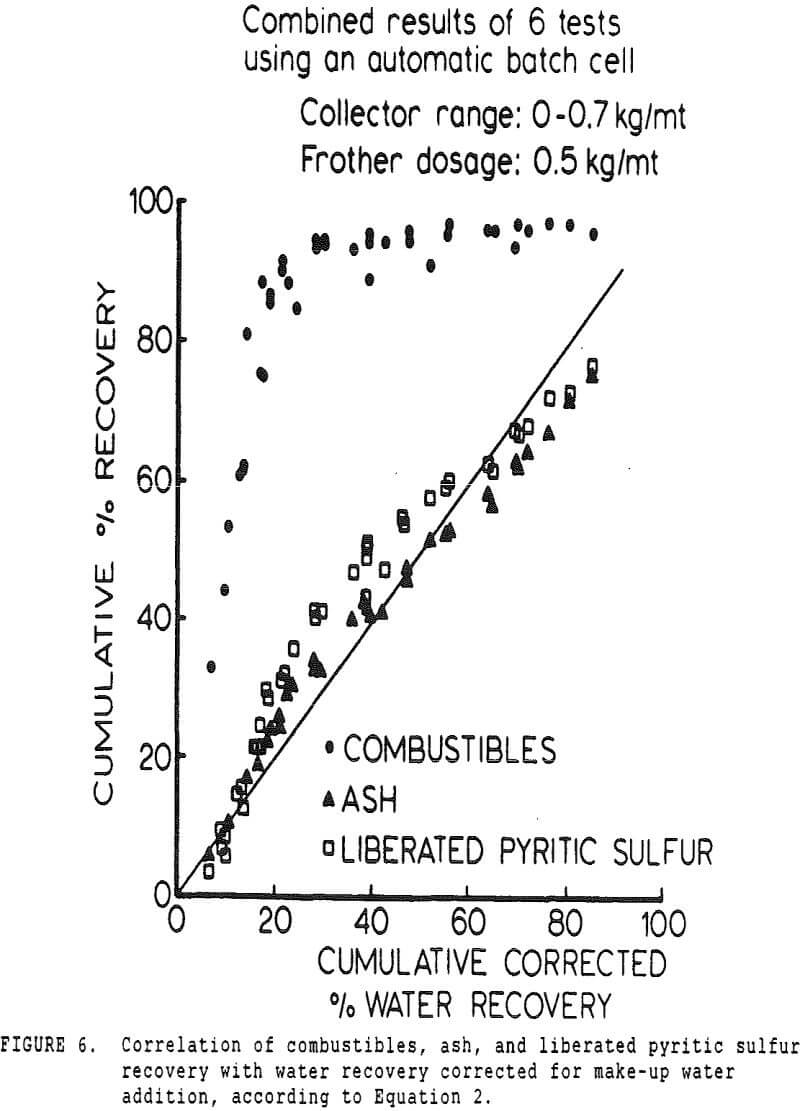
A second correction was made necessary by the constant-level feature of the conventional cell. While a constant froth depth is needed to reduce variations in the entrainment constant, the addition of make-up water makes the cell continuous with respect to water, while remaining batch with respect to solids.
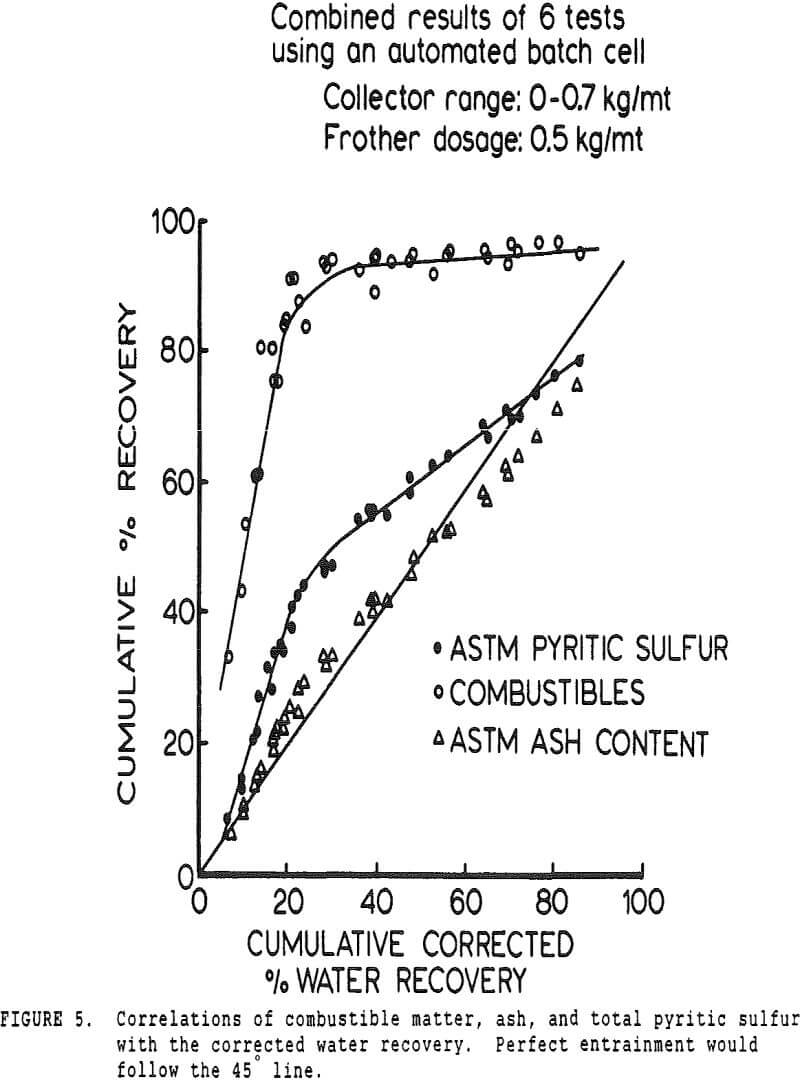
The pyritic sulfur behaves in an intermediate fashion, with recovery being initially slightly faster than that of water, then abruptly becoming slightly slower. It is of interest to note that, within the limits of accuracy of the data, this inflection occurs at precisely the same water recovery as the abrupt slope decrease in the combustibles curve, which occurs due to exhaustion of the floatable coal particles in the cell.
Unfortunately, there does not appear to be any available method for conclusively measuring the relative quantities of locked and liberated pyrite particles in coal flotation, nor is there an effective method for isolating coal-free pyrite from coal which does not also either contaminate or oxidize the pyrite surfaces.
If it is assumed that pyrite is indeed primarily recovered by locking and entrainment, then it should be expected that sulfur rejection would be poorest for the finer coal sizes. This is a result both of entrainment being most important for the fine particles due to their low settling rates, and of the low solids content of fine-particle flotation froths, which increases the quantity of entrained water for a given solids recovery.