Table of Contents
In recent years, coal research has been directed toward environmental problems of the electric power generation industry. A major problem of coal-burning power-plants is reducing the air pollutants in stack gases. In most of these plants, the chief pollutant is SO2, which results from the combustion of sulfur compounds present in the coal.
Stack-gas cleaning systems are expensive to install and operate and in some cases would not be needed if most of the sulfur-containing components were removed from the coal prior to combustion.
The source of nearly 50 pct of the sulfur content of an average bituminous coal used by an electric utility is pyrite (FeS2); pyrite is a component that is not chemically bound to the coal and, therefore, can be removed by physical separation methods. Removing 50 to 70 pct of the pyrite would accomplish only a 25- to 35-pct reduction in SO2 emission. However, in coals in which the sulfur content is chiefly pyritic, pyrite removal would reduce SO2 emission much further. Furthermore, recently proposed emission standards from the Environmental Protection Agency for new stationary power sources limit SO2 emission to 1.2 lb/10 6 Btu for coal-fired units. This standard means that without a stack-gas treating system, the permissible total sulfur in a 13,000-Btu/lb coal is only 0.8 pct. Even when treating the stack gas with a lime slurry system that removes 73 pct of the, permissible sulfur level in the coal is only 3 pct. Many coals of Appalachian and Midwest regions would require prior pyrite removal treatment to meet this sulfur restriction.
Pyrite in lump form can be removed from relatively coarse coals by presently used wet-cleaning methods at the mine-site preparation plants. However, the fine pyrite particles cannot be removed until the coal is finely pulverized. Because of transportation losses, the coals are not usually ground to this fine size until they have reached the point of use. Furthermore, should the finely ground coal be cleaned by wet methods, problems of coal drying and disposal of wet refuse would be introduced.
An additional reason for removing pyrite prior to burning is conservation of the mineral value. In 1969, over 300 million short tons of coal was burned by electric utilities in the United States. If it is assumed that these coals averaged 2 pct pyrite, 6 million tons of pyrite (53 pct sulfur-47 pct iron) was converted to SO2 and iron compounds. The iron portion of this pyrite amounts to about 2.0 pct of the domestic steel production in 1969, and the sulfur portion amounts to nearly 35 pct of the domestic sulfur consumption in 1969. It would be easier to recover the sulfur and iron from a stockpile of dry, pyrite-enriched reject than from wet reject or from combustion products.
Related Work
Air classifications have been used in mineral-processing industries for several years. Air tabling is used to separate materials by particle size, shape, and specific gravity. Centrifugal separators are used in other processes to dedust a larger aggregate or to separate a ground material into small and large particle-size fractions. Previous work by the Bureau of Mines made use of centrifugal separation to concentrate pyrite from closely sized fractions of coal. For several years, electrostatic separations have likewise been used in concentrating ore and separating material. Electrostatic separation units with rotor lengths up to 10 ft that are capable of processing 4 tons/hr are used for concentrating iron ores. Relatively recent developments in electrostatic separation technology that can be applied to coal include the following: (1) The use of a rectified, high-peak pulsed current at a choice of frequencies as the charging current to the field electrode, and (2) the use of an arcless electrode to eliminate the explosion hazard when a material such as fine coal is treated. Arcless electrodes are available for smaller units. Another reference of interest is the work of Fraas in which he describes the development of the electrostatic method of particle separation.
Experimental Work
Dry-Separation Process
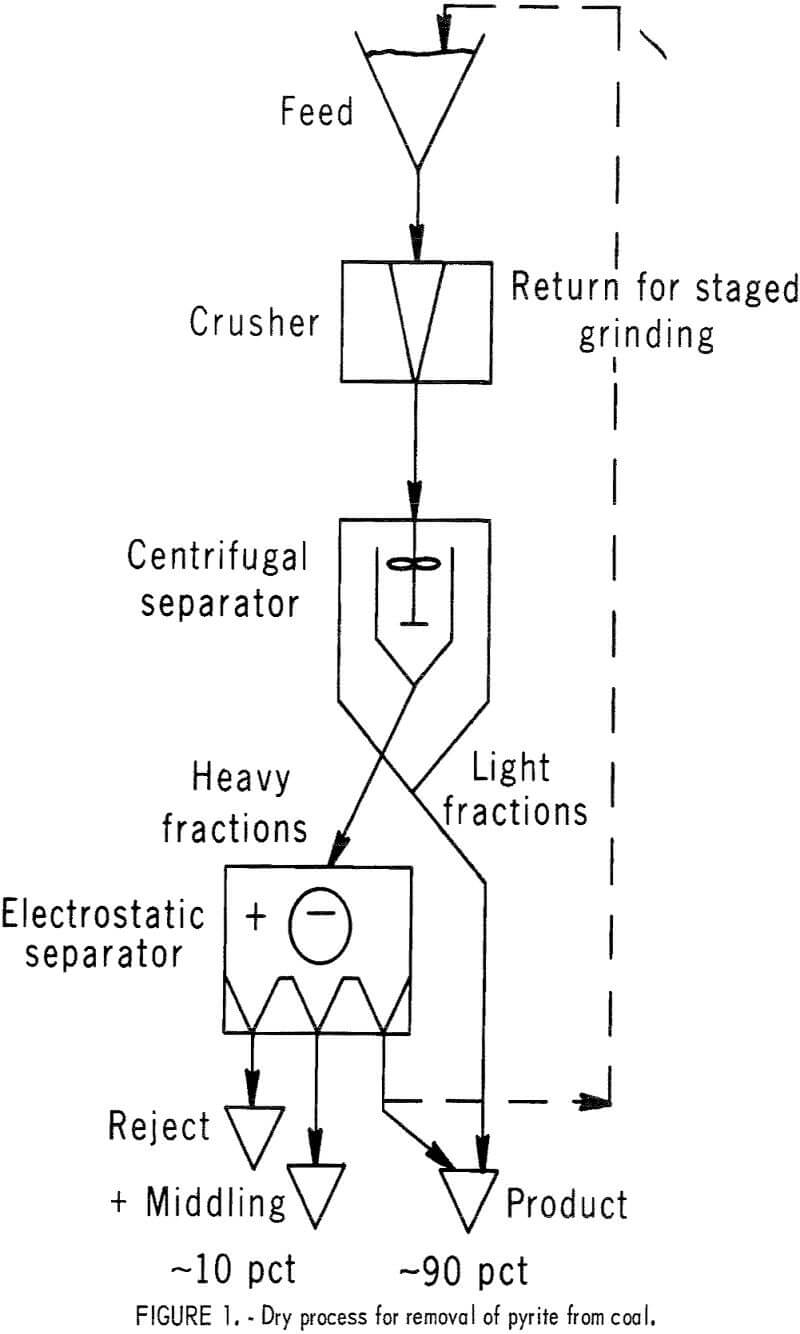
The two pyrite-separation methods (centrifugal and electrostatic) that constitute the dry process supplement each other beneficially. By removing most of the lightweight, pyrite-depleted portion in the centrifugal step, electrostatic separation of pyrite from the remaining pyrite-enriched heavy portion is more effective. The dry process reported here is shown in figure 1. The feed is pulverized, then taken to centrifugal separation to remove the fines as lightweight, pyrite-depleted fractions, after which a heavier pyrite-enriched portion remains. The accumulated light fractions, which amount to 10 to 50 pct of the feed, are taken as a clean product, and the remaining heavy fraction is passed to electrostatic separation in which the pyrite is concentrated in a reject of about 10 to 15 pct. For some coals, separation may be considerably improved by stage grinding; that is, by removing pyrite at intervals as the coal is pulverized to its final size.
Centrifugal Separation
Since centrifugal separation depends on particle mass as one of the criteria of separation, it might not distinguish between a relatively large pyrite-free particle and a relatively small, pyrite-rich particle of the same mass. Therefore, for efficient separation, steps must be taken to avoid too wide a range of particle sizes reaching the centrifugal section at any one time. In the first step, the coal is pulverized and fed to the centrifugal separator (fig. 2) with the entraining air set at a relatively low velocity. As the coal falls through the hollow drive shaft onto the spinning spreader disk, only those particles light enough to become entrained in the upward current of air will be carried to the centrifugal fan. Here the heavier, pyrite-rich particles are thrown to the chamber wall where they fall from suspension in the static air layer next to the wall and leave the separator via the heavy-product exit. The remaining entrained particles are carried into the annular space between the inner and outer cones where they fall from suspension and leave the separator via the lightweight product exit. The portion of the coal that was too heavy to become entrained as it was thrown from the spinning spreader disk falls from the separator as a heavy product. In the second step, the velocity of entraining air is increased, and the heavy product is resubmitted to the centrifugal separator in which a second light
portion is removed. This procedure is repeated (usually three or four times) until the accumulated lightweight portions equal about 75 pct of the minus 200-mesh content of the coal. In practice, rather than refeed the heavy
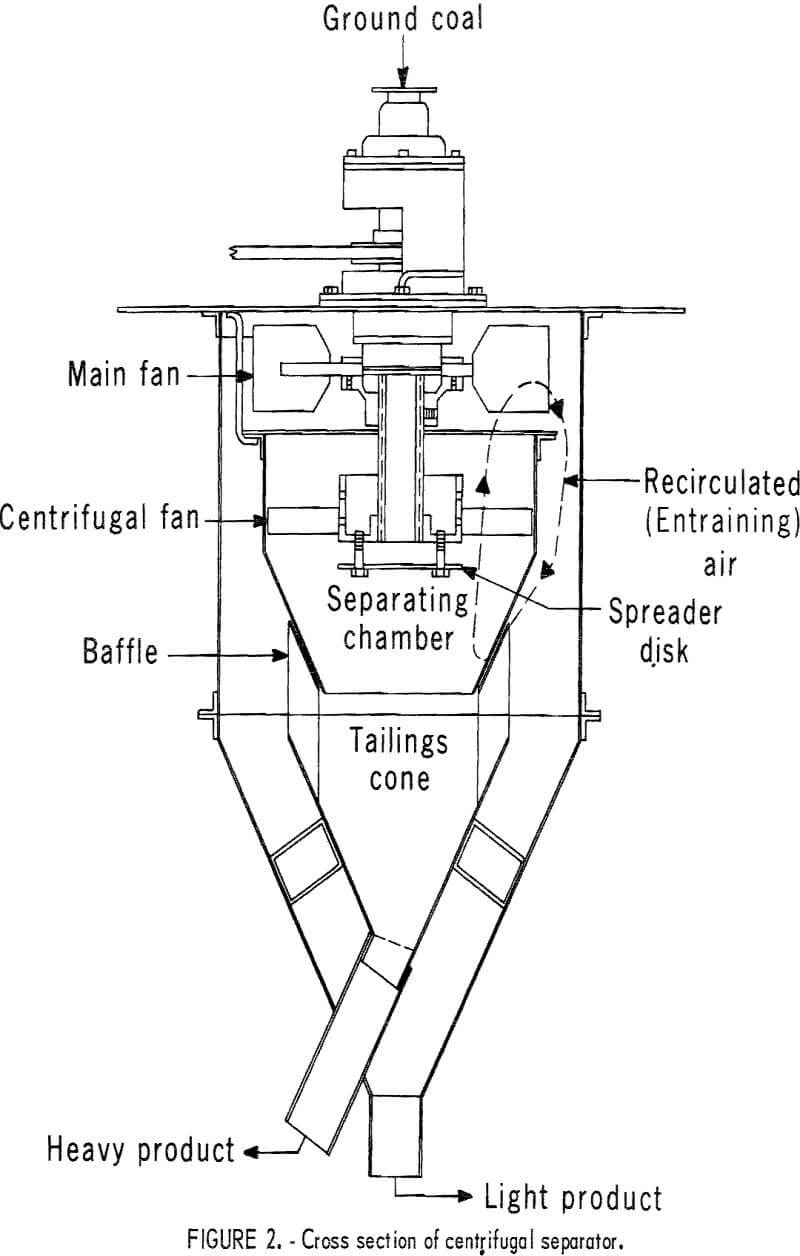
product several times to remove a series of light portions, the coal would probably be run through a series of separators with entraining air velocity increasing from separator to separator.
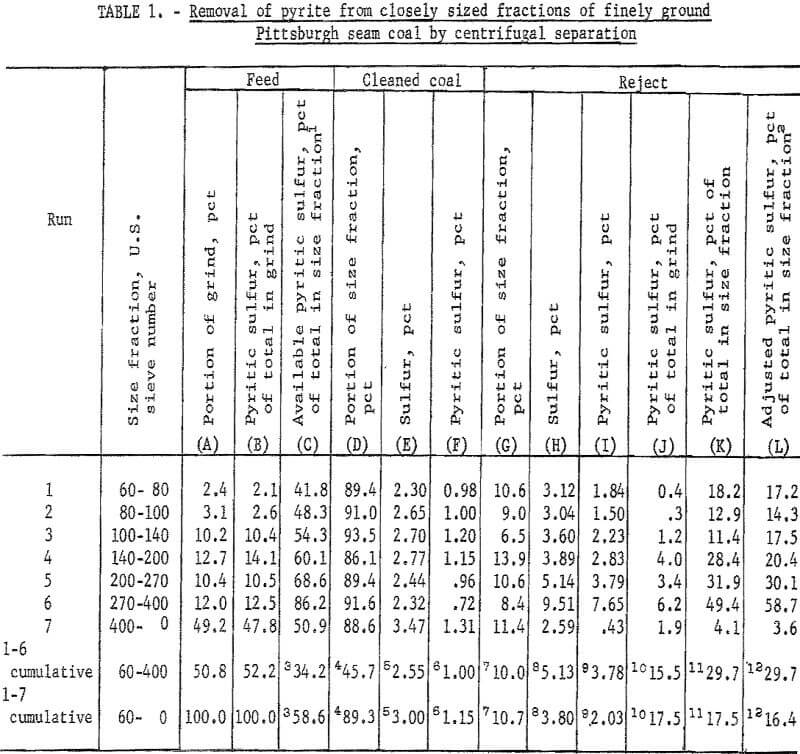
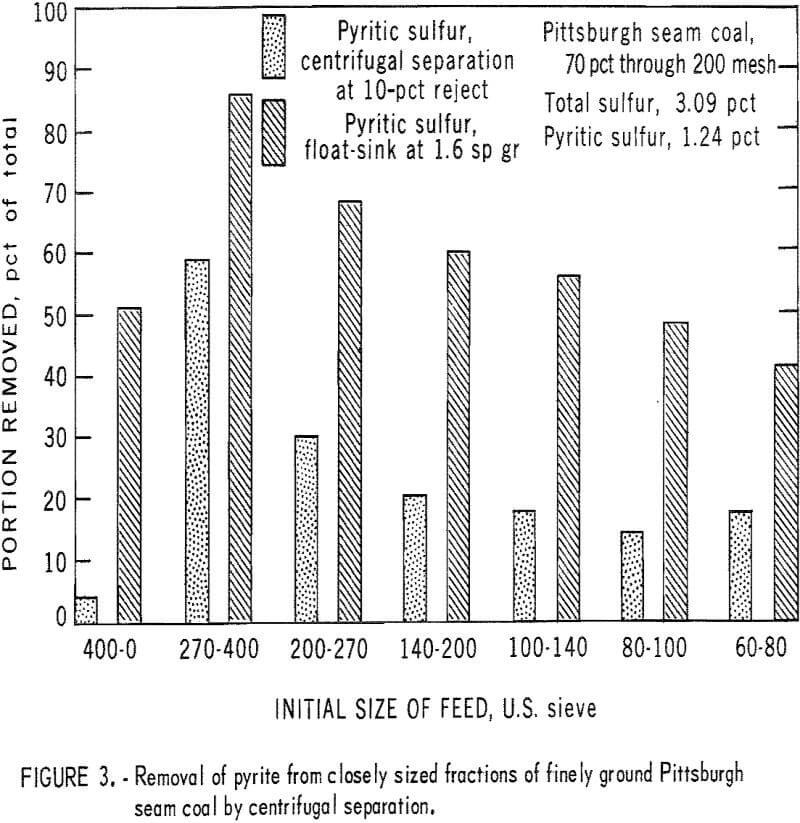
The data in table 1 and figure 3 show the results of an experiment (not suggested as a part of a practical dry separation process) to test the capability of centrifugal separation for removing pyrite from closely sized fractions of a Pittsburgh seam coal crushed to fine particle size in an air-swept bowl and ring mill. The bar graph of figure 3 demonstrates that both the pyritic sulfur in a 10-pct reject taken by centrifugal separation and the pyritic sulfur that is separable by float-sink separation at 1.6 sp gr increase as the particle size decreases from 60 by 80 mesh to 270 by 400 mesh. In the 270- by 400-mesh fraction, nearly 60 pet of the pyritic sulfur in the fraction is concentrated in a 10-pct centrifugal reject, and about 85 pct is available by float-sink separation. It is significant that the 400- by 0-mesh fraction underwent reverse enrichment in centrifugal separation. The behavior of the 400- by 0-mesh fraction demonstrates the wide range of particle size and the extreme fineness of pyrite in this fraction. The amount of extremely fine pyrite in this fraction probably was increased by the grinding action of an air-swept mill in which heavy particles are returned to the mill until fine enough to be carried out by the air sweep.
Electrostatic Separation
Electrostatic separations utilize the difference in conductivity or dielectric properties of coal and associated material, such as pyrite, to maintain or dissipate an induced charge under dynamic conditions. Figure 4 shows the separation section of a “high-intensity” electrostatic separator. A dispersed layer of coal is fed onto the electrically grounded rotor. The particles pass under an active electrode for exposure to ionic bombardment. Subsequently, the conductive pyrite-rich particles become equipotential with the rotor and are released. The coal-rich dielectric material remains attached to the rotor. All particles are also subjected to centrifugal and gravitational forces. The splitters are used to divide the stream of released material for the most effective concentrations of reject, middlings, and product.
Dry feed materials subjected to a high concentration of polar charge from a corona field of dissociated gas molecules are momentarily attracted to the rotor. This high-intensity band is relatively narrow and depends on the promixity of the active electrode and the voltage of the rectified transformer current. A range of 18 to 30 kV was found adequate for particle attraction although the unit had a range of 18 to 40 kV. Generally, fine particles separate more effectively with high rotor speeds and lower voltage, and larger particles give better separation at lower rotor speeds and high voltages. For these reasons, the size of particulate is best kept within narrow ranges for a single pass.
Efficient separations are also affected by other factors, such as atmospheric humidity and the moisture and fines content of the coal. Humidity affects ionization, and separations are improved at midrange (about 50 pct). Moisture and fines content of the coal affect the extent of balling of fines and coating of larger particles with fines. Coating of particles and grounding surfaces with fines, whether caused by static attraction or by excessive moisture, alter the selective grounding necessary for effective separation and must be controlled. The moisture content of the coals used in this work ranged from 1.5 to 3 pct.
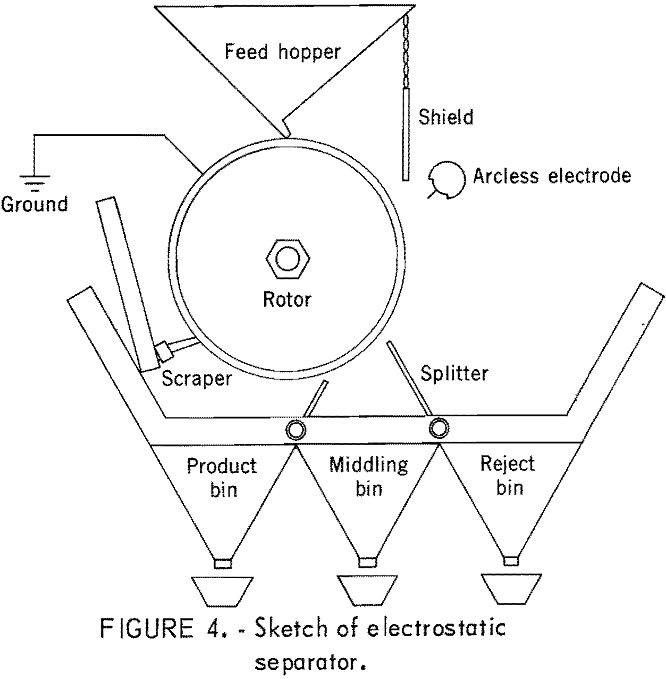
The heavy portion of coal that remains after removal of the desired amount of light product by centrifugal separation is submitted to electrostatic separation. If the feed contained considerable amounts of plus 50-mesh material, it was screened into plus 50-mesh and minus 50-mesh fractions. Each fraction was processed separately using the voltage, rotor speed, and divider setting that gave best results from a visual evaluation of the reject and product. Generally, the larger size fraction was run at slower rotor speed and higher voltage. Atmospheric humidity was maintained at about 50 pct.
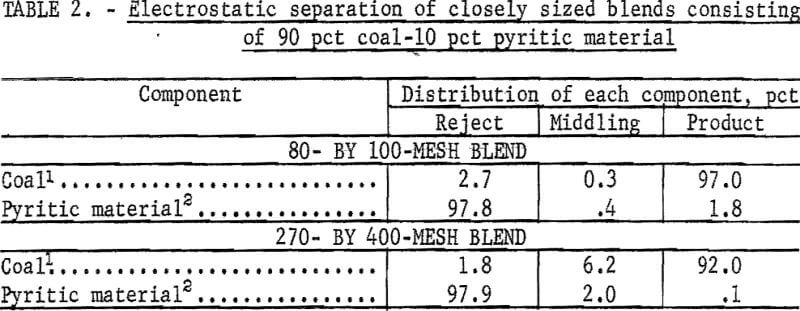
Although such conditions are not envisioned as a part of a working dry-separation process, an experiment to test the effectiveness of electrostatic separation for concentrating pyrite from two synthetic coal-pyrite blends is shown in table 2. Each component of the blends had been previously sized to 80 by 100 mesh or 270 by 400 mesh and then cleaned by float-sink separation; that is, the coal floated at 1.6 sp gr, and the pyrite material sank at 2.89 sp gr. The results obtained by submitting these blends to electrostatic separation show that separation was very sharp. With the 80- by 100-mesh blend, nearly 98 pct of the pyritic material was rejected, and 97 pct of the coal went to the product bin. Treatment of the 270- by 400-mesh material placed 98 pct of the pyrite in the reject bin, 92 pct of the coal in the product bin, and about 6 pct of the coal in the middling bin. This experiment demonstrated the effectiveness of electrostatic separation on closely sized materials of markedly different dielectric properties.
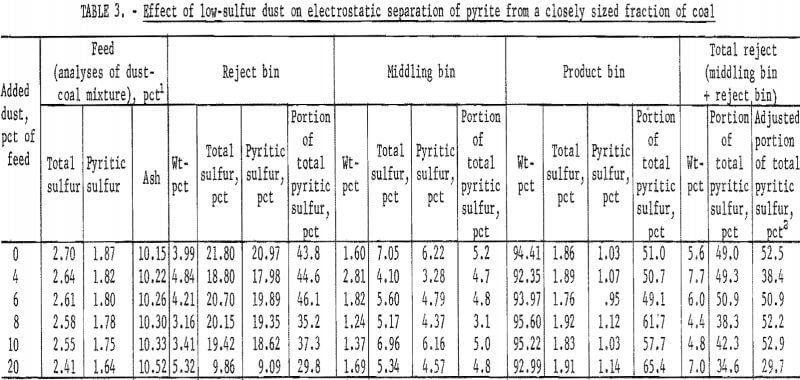
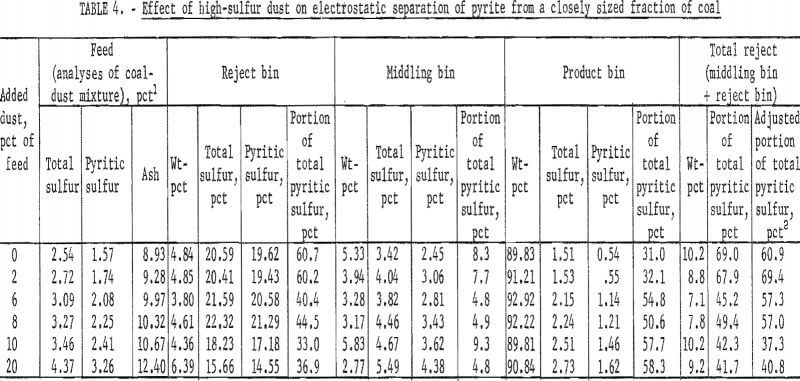
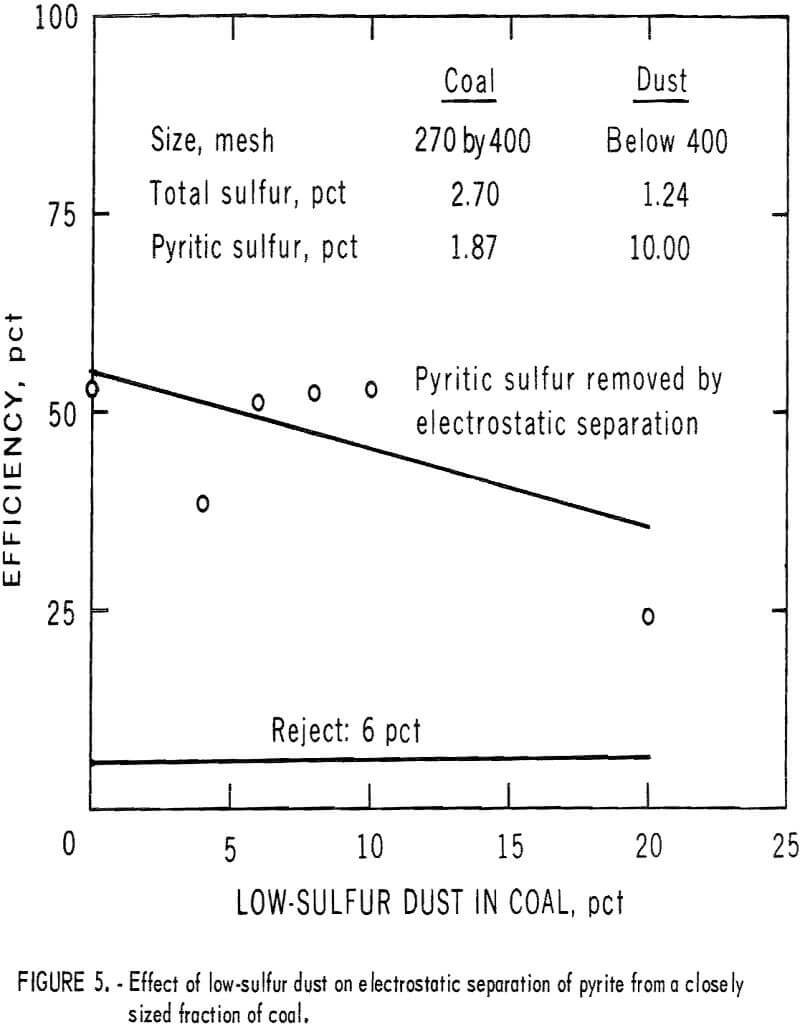
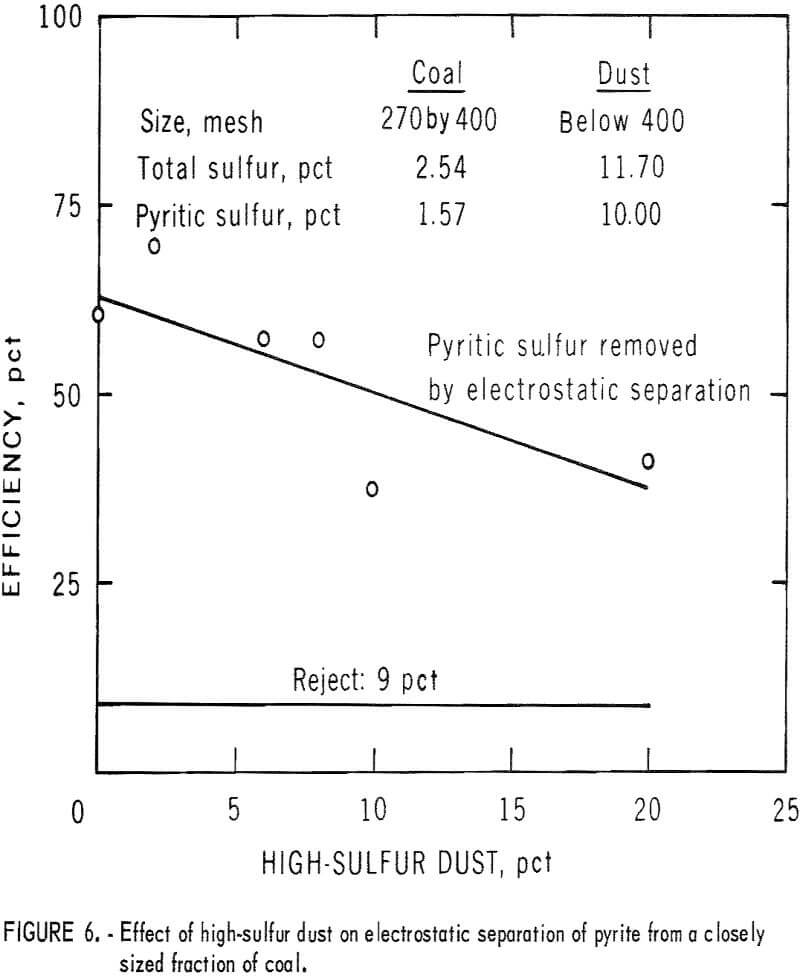
The effect of dust on the efficiency of electrostatic separation of pyrite from a closely sized coal is shown in table 3 and table 4 and the accompanying graphs (figs. 5-6). Figure 5 shows the results with a low-sulfur dust. Separation efficiency is not significantly decreased until more than 10 pct dust is added. Because the dust is of low-sulfur content, misplaced dust does not contribute greatly’ to the pyritic sulfur content of the reject or the product. Figure 6 shows the effect of adding a high-sulfur dust.
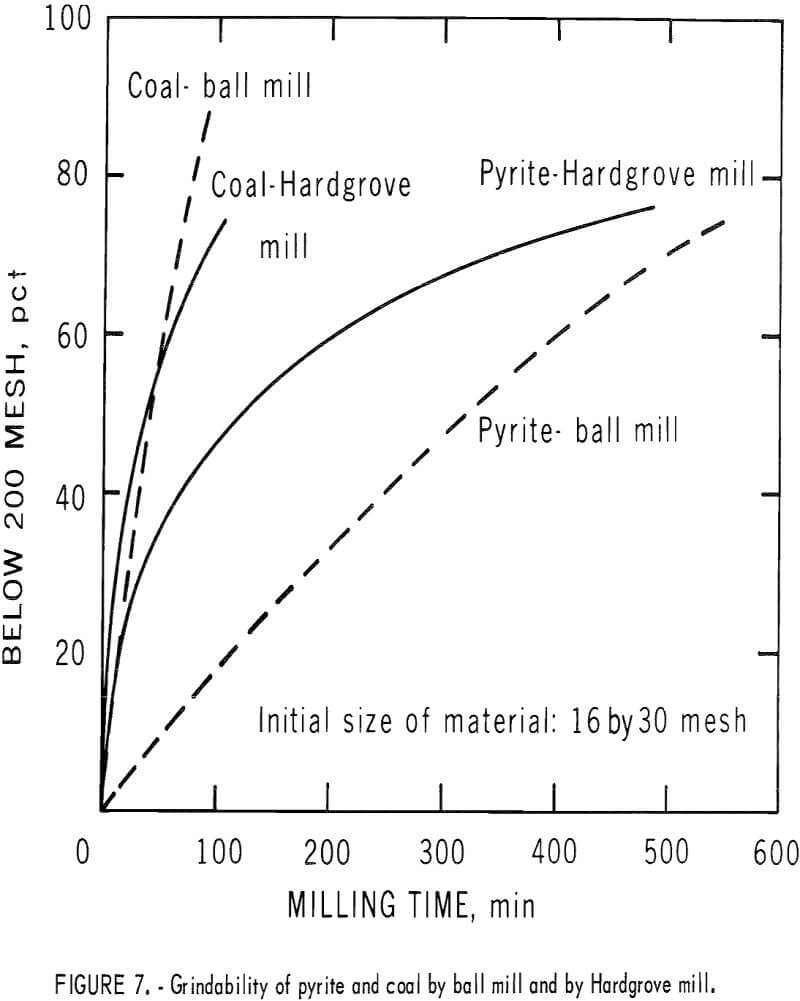
Grindability of Pyrite and Coal-An Aid in Separation
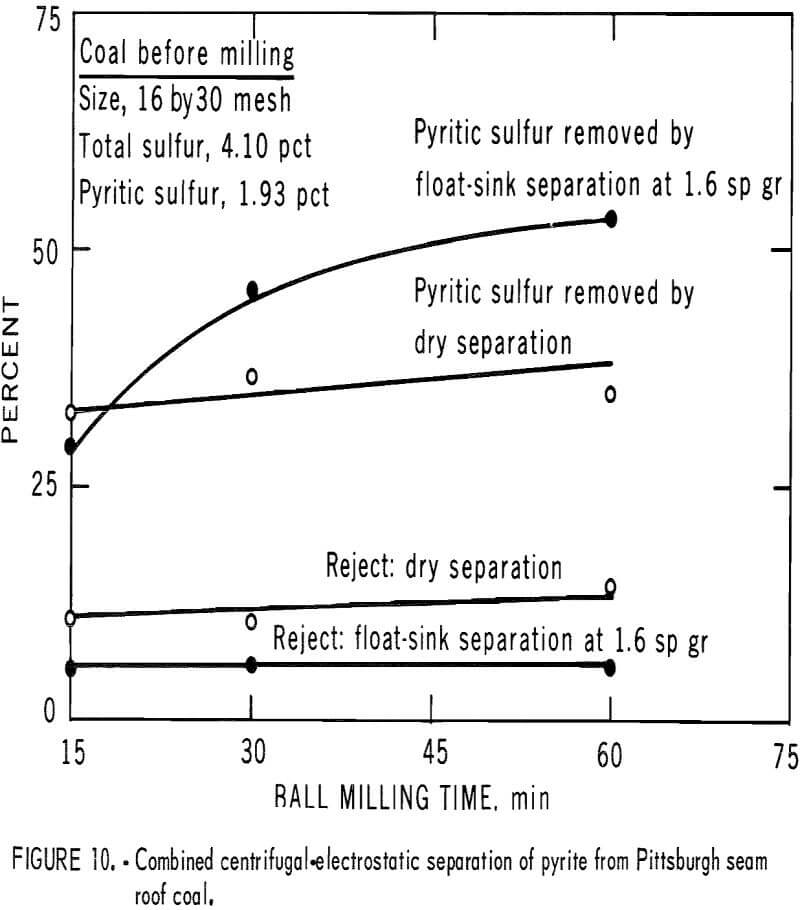
Figure 7 demonstrates the behavior of coal and pyritic material when subjected to two types of grinding action. The materials used in this test had been previously sized to 16 by 30 mesh, then cleaned by the float-sink separation (the coal floated at 1.6 sp gr, and the pyritic materials sank at 2.89 sp gr). The results show that in ball milling, coal is ground to 70 pct below 200 mesh in about one-seventh the time required for the pyrite. Although the spread is less in the early stages of Hardgrove milling (ball and race milling), it increases rapidly as milling proceeds. Because both centrifugal and electrostatic separation remove larger particles of pyrite more efficiently than does fine-size particles, the advantage of pulverizing in a manner that minimized pyrite grinding is apparent. Thus, if the grinding is incorporated as stage steps in the pyrite-removal system, pyrite can be removed as it is released from the coal matrix.
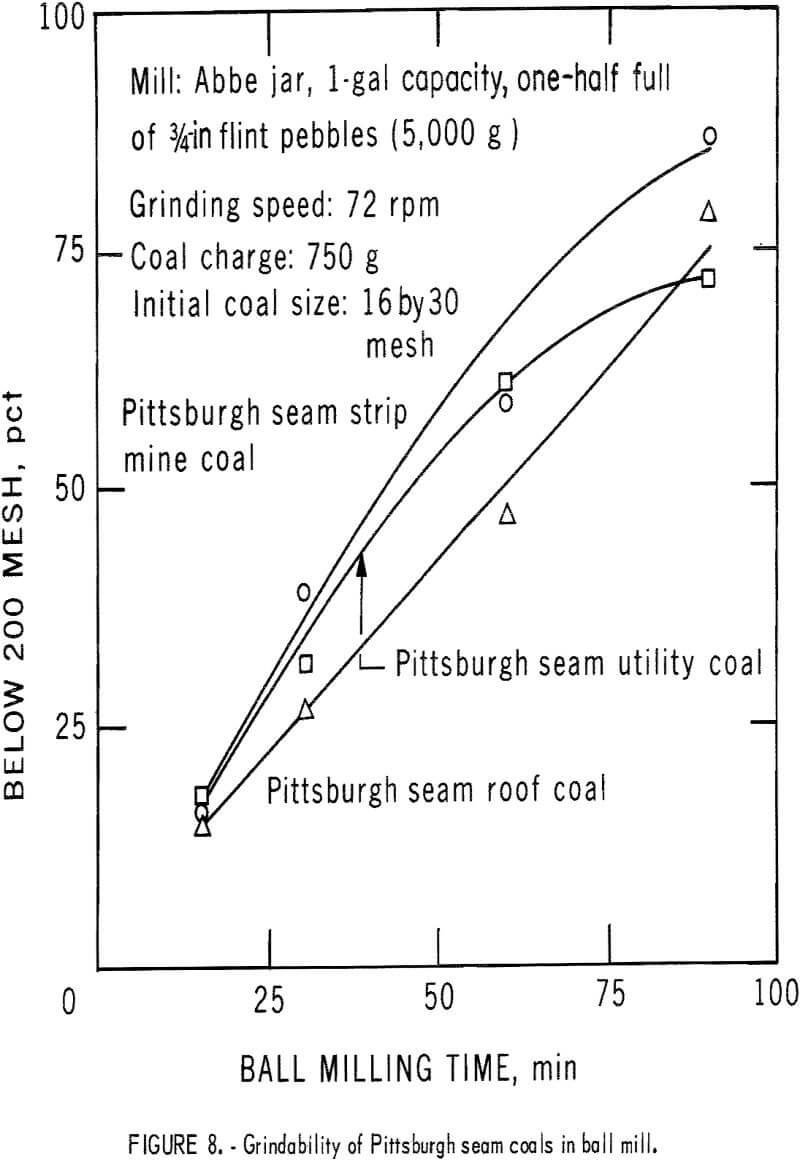
Combined Centrifugal-Electrostatic Separation
Three Pittsburgh seam coals were cleaned by the dry-separation process. The first of the coals, a roof coal from a mine in northern West Virginia, was high in both organic and pyritic sulfur. The second, a utility coal from northern West Virginia, was representative of the utility coals burned in the tristate area around northern West Virginia; the third coal, from a strip mine near the West Virginia-Ohio border, was chosen because of its relatively high pyritic sulfur content.
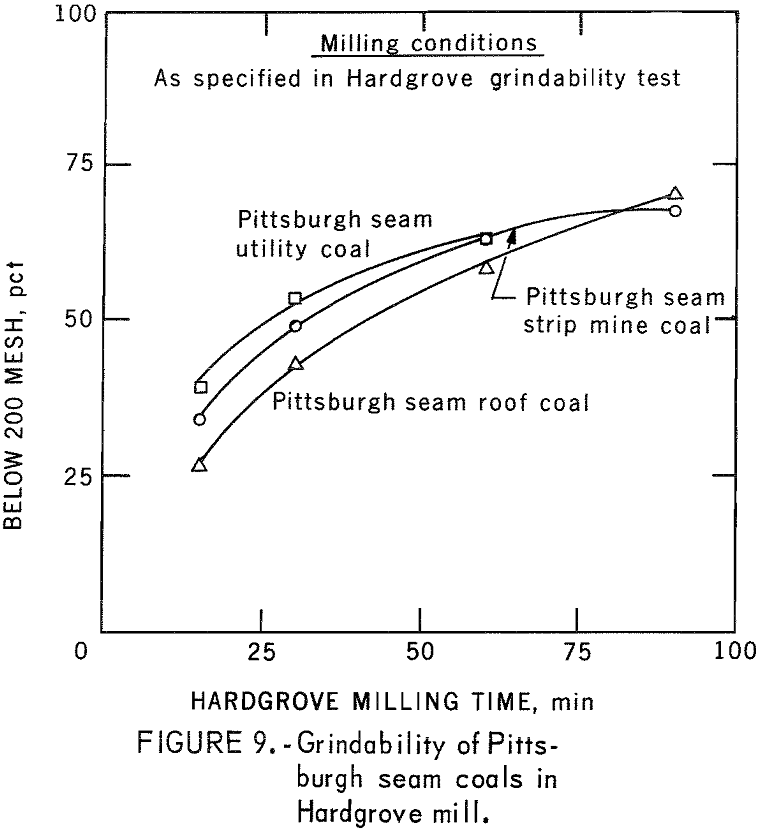
The behavior of these coals when pulverized by ball milling and Hardgrove milling is shown in figures 8-9. Ball milling produced a greater size spread between the coals in stages of grinding than did the Hardgrove mill.
Float-sink behavior of these coals was determined by stirring and shaking the pulverized coal in a separatory funnel with a liquid of 1.6 sp gr until no further sink material appeared. With the finely pulverized coal grinds, as long as 72 hr was required to complete a separation.
After establishing a total sulfur versus pyritic sulfur relationship for each of these coals over the range of sulfur concentrations met in the separations, pyritic sulfurs were determined from total sulfur analysis by these relations.
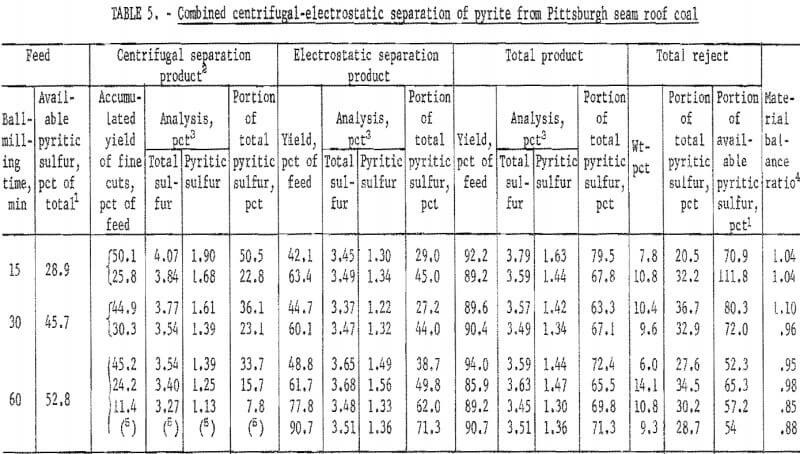
The results of dry separation from the Pittsburgh seam roof coal are shown in table 5 and figure 10. In table 5, the results of two or more separations on each of the three grinds are shown to demonstrate that removal efficiency is affected by the amount of centrifugal product taken. Results were
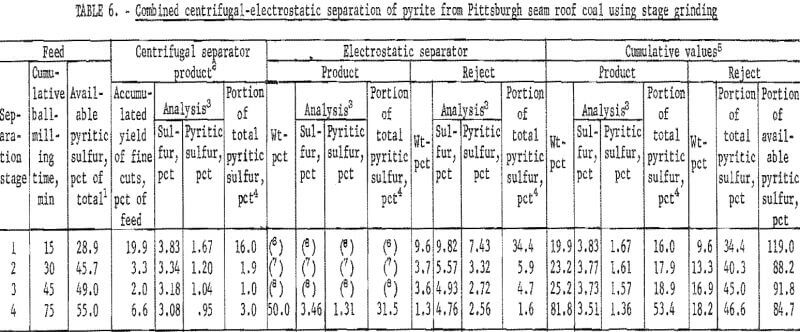
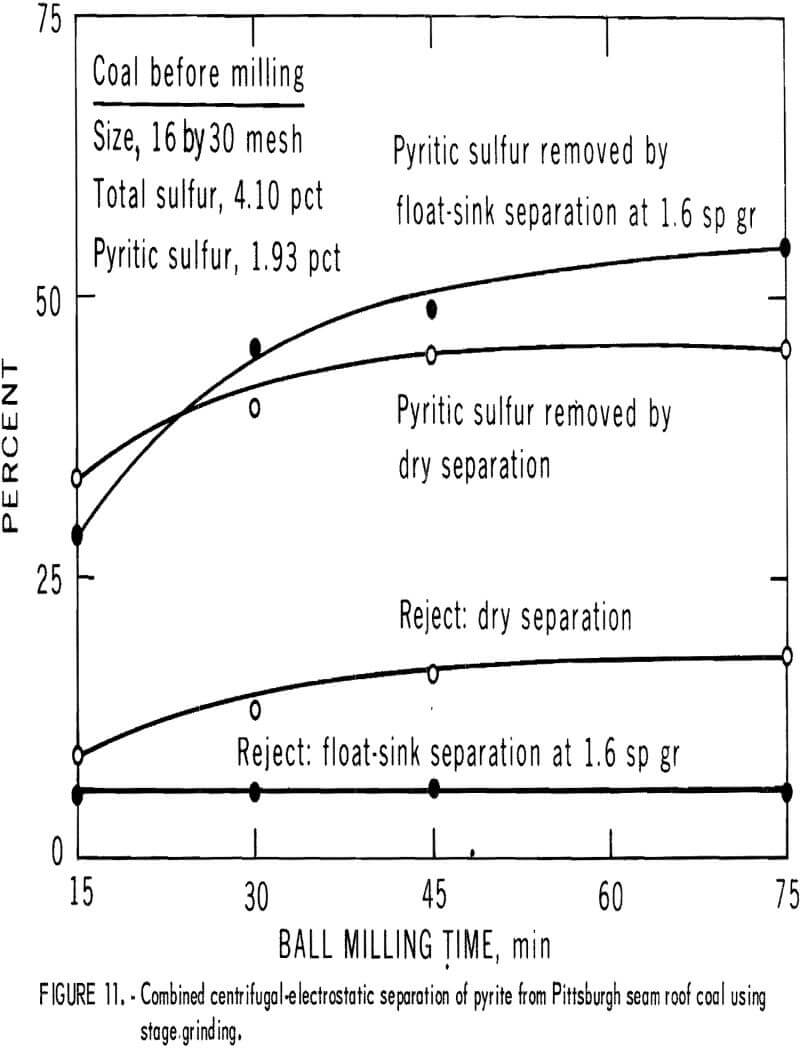
best when about 25 to 35 pct was removed in the centrifugal separator. The best separation from each grind is plotted in figure 10. About 35 pct of the pyritic sulfur, which amounted to about 70 pct of the available pyritic sulfur, was removed by dry separation. Table 6 and figure 11 show the results of dry separation on the same coal when grinding is accomplished in stages with pyrite-removal treatment between stages. About 45 pct of the pyritic sulfur, which amounted to about 90 pct of the available pyritic sulfur, was removed. Stage grinding improved pyrite removal by about 30 pct and increased the reject portion from 12 pct to 18 pct.
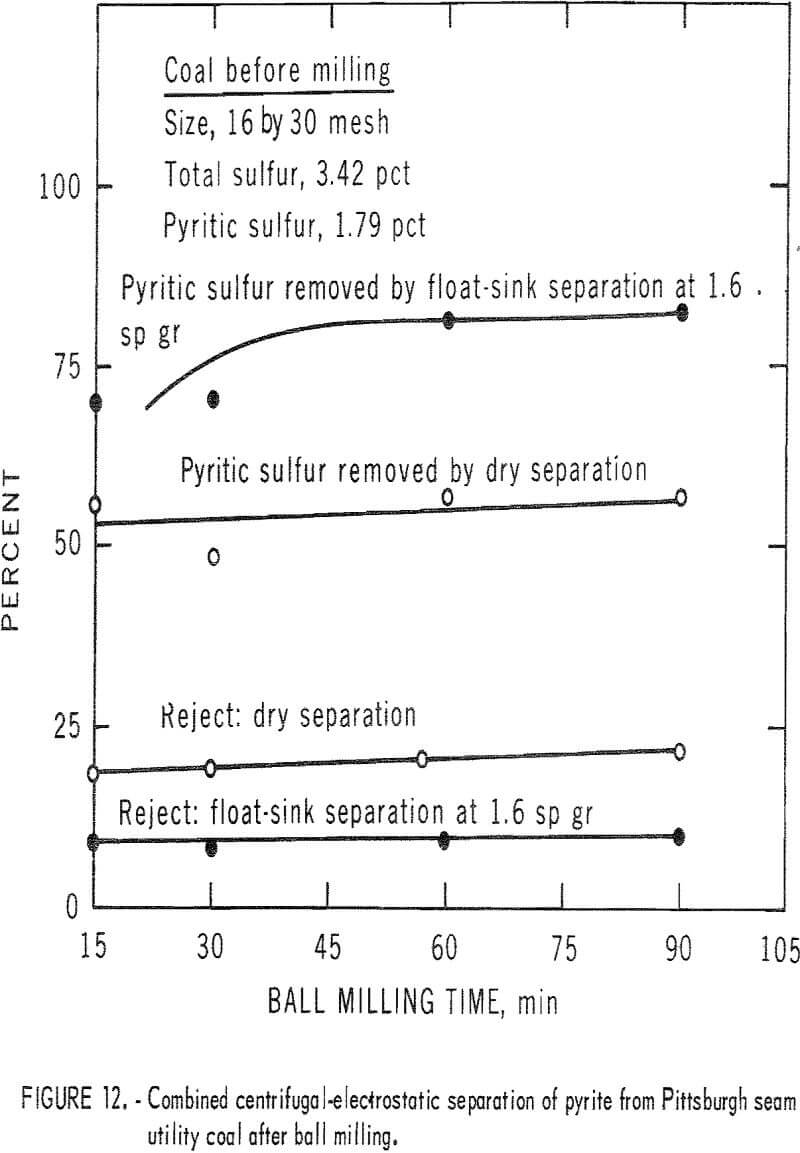
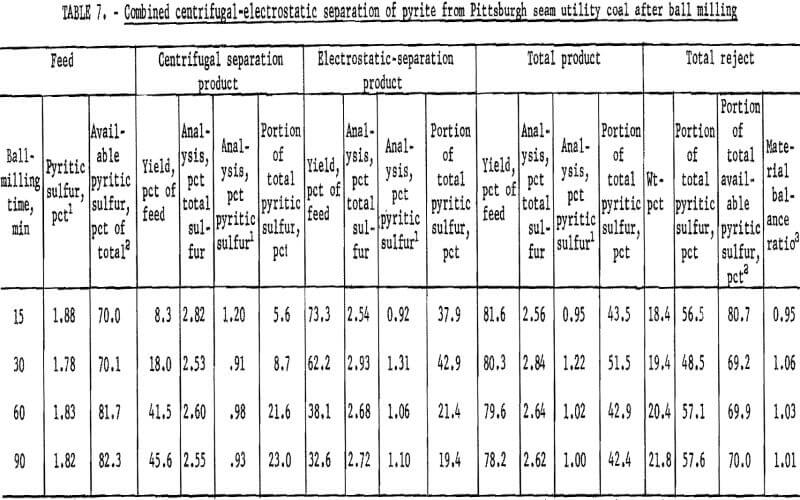
A second coal from the Pittsburgh seam that was representative of the utility coals burned in this area was crushed by ball milling and Hardgrove milling, and each pulverized consist was cleaned by dry separation. The results for the ball-milled product are shown in table 7 and figure 12. Nearly 60 pct of the pyritic sulfur was removed in rejects of about 20 pct. This amounted to about 70 pct of the available pyritic sulfur. The corresponding results with the Hardgrove-milied product are shown in table 8 and figure 13. About 55 pct of the pyritic sulfur, which amounted to 65 pct of the available pyritic sulfur, was removed in rejects of slightly over 20 pct. The separability of pyrite from the ball-milled product and the Hardgrove-milled product was essentially equal for this coal.
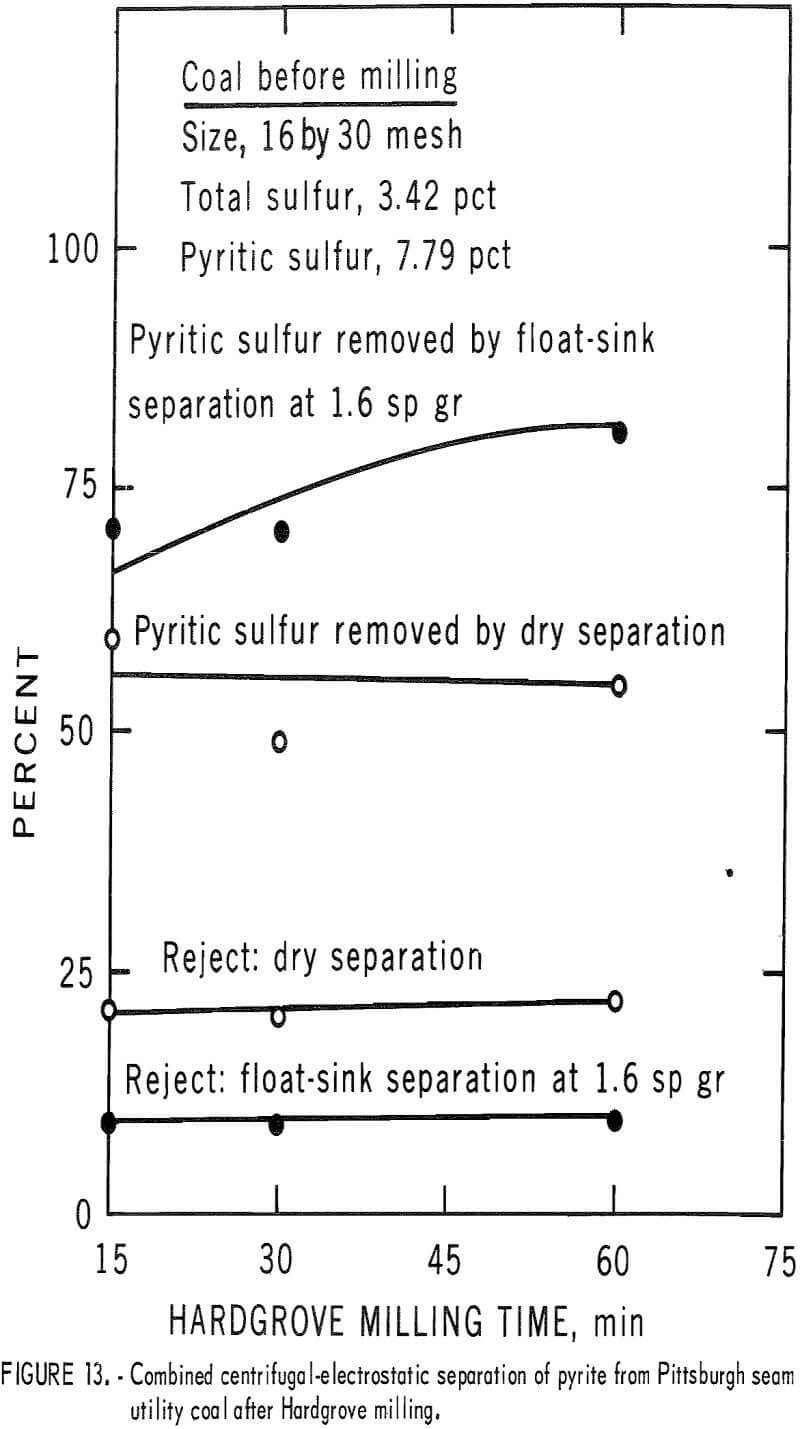
The third coal from a Pittsburgh seam strip mine was chosen for testing because of its relatively high pyritic sulfur content, much of which occurred as a larger, more available particulate. Pulverized products from ball milling and Hardgrove milling of this coal were tested. The ball-milled
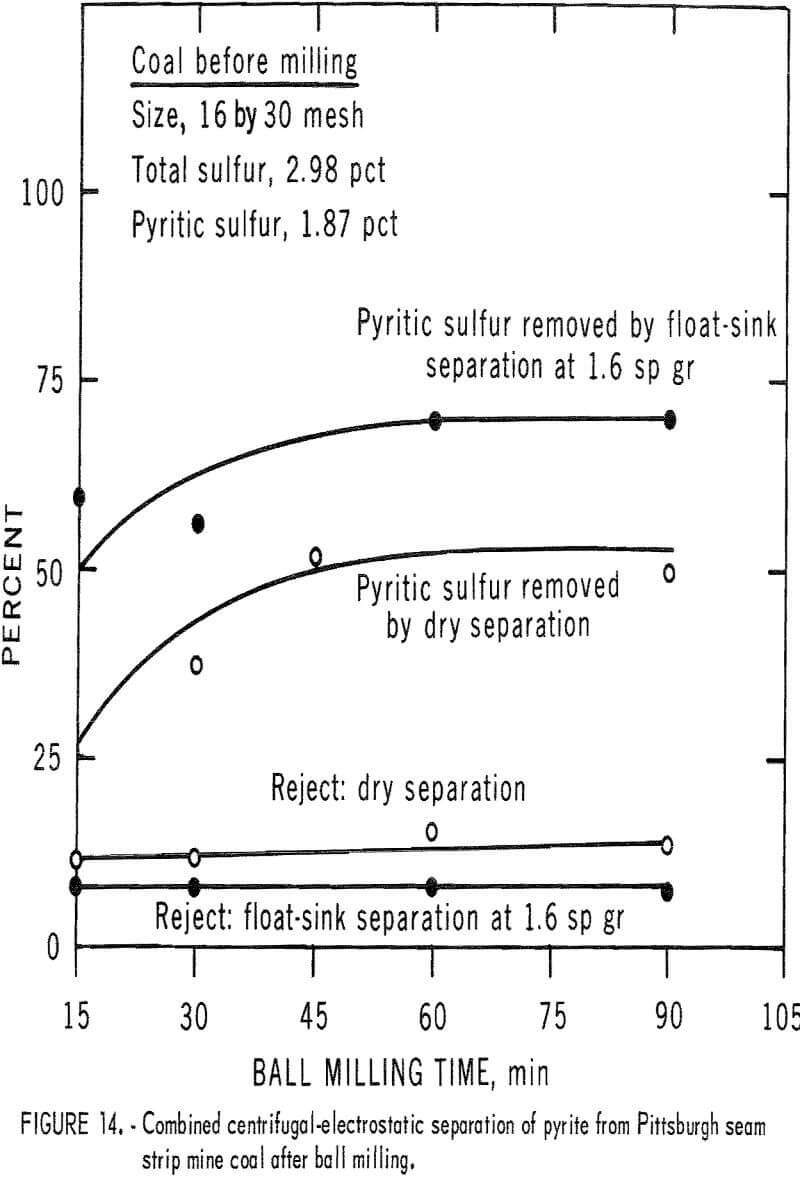
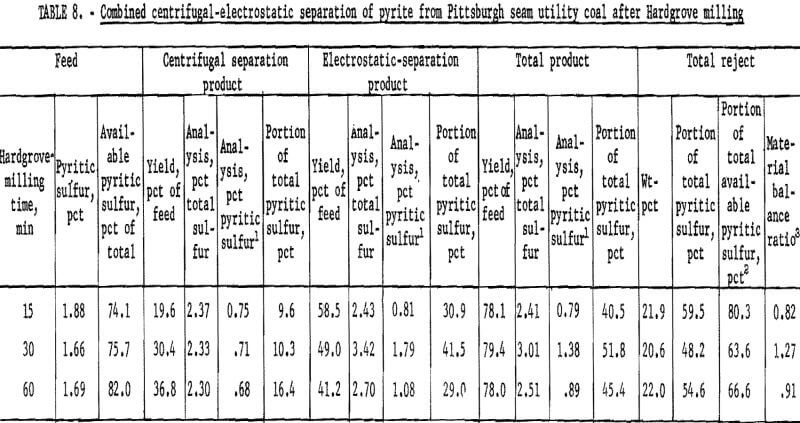
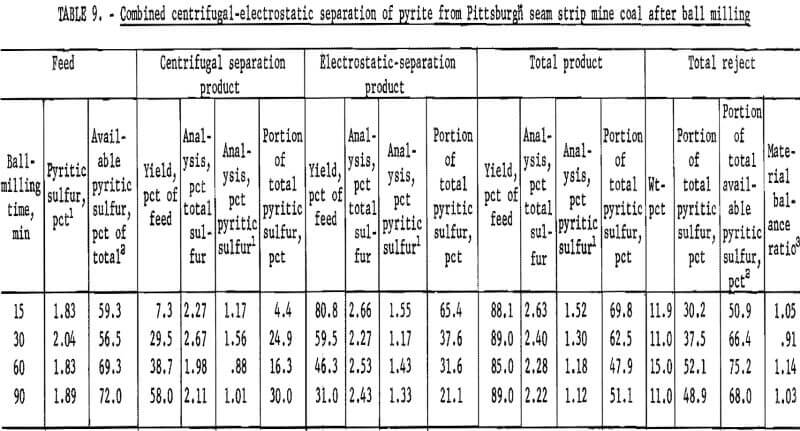
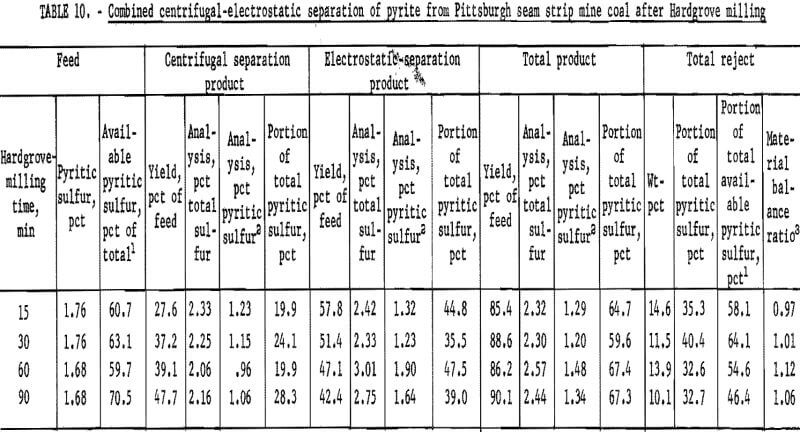
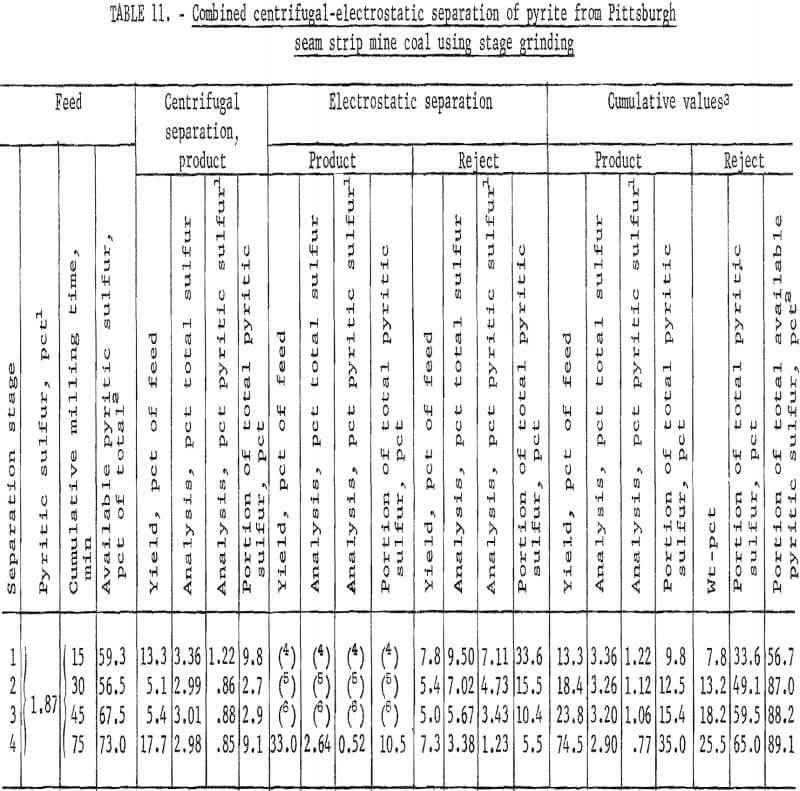
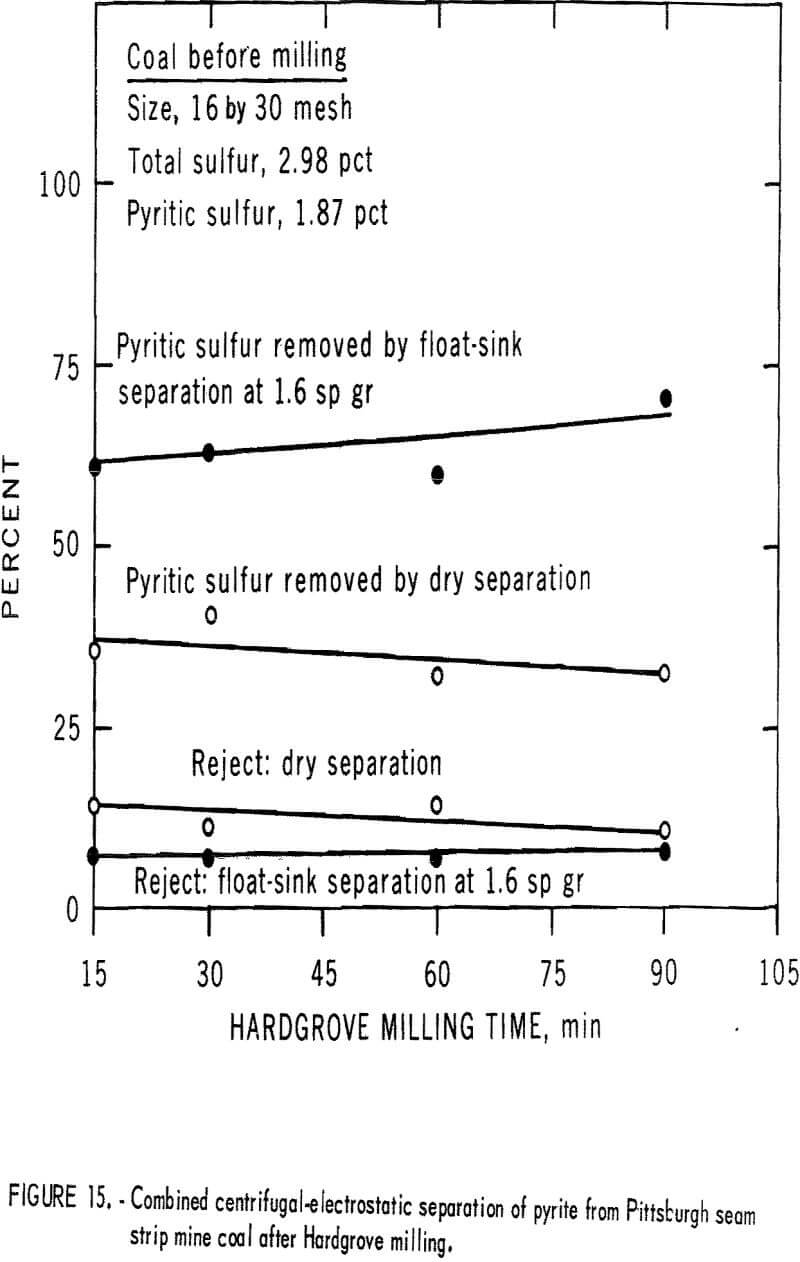
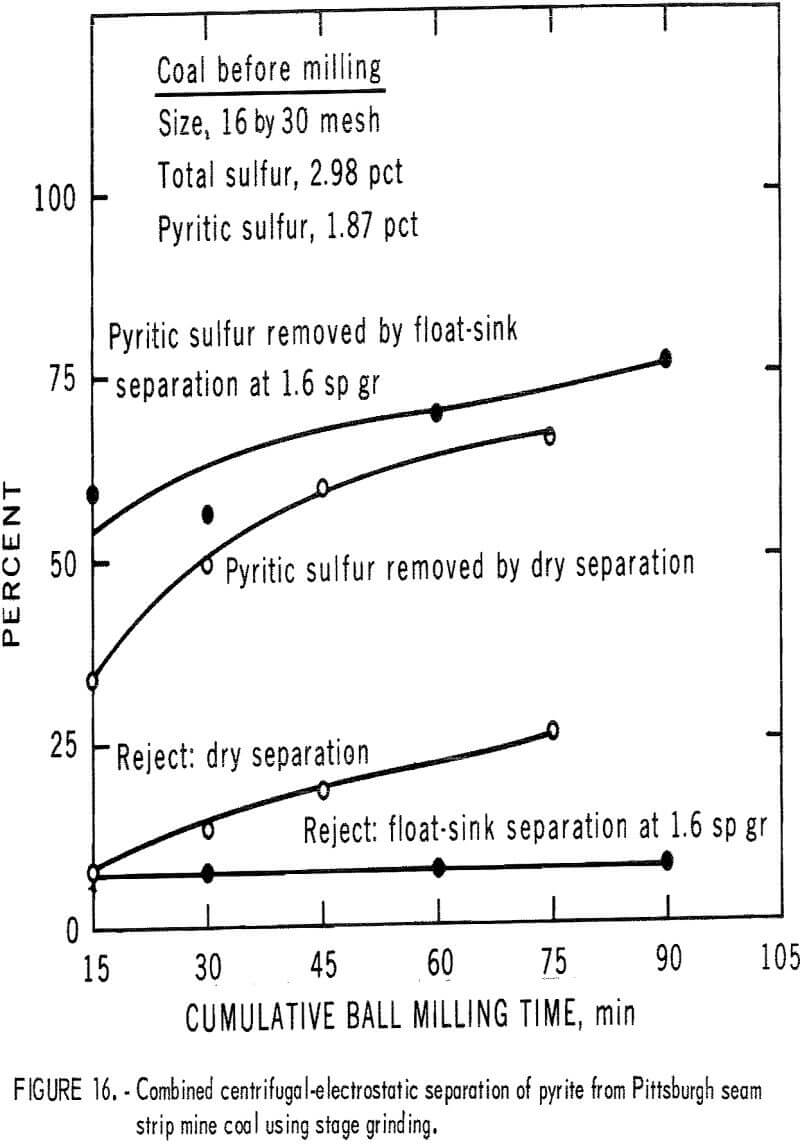
products were more amenable to dry separation than were the Hardgrove-milled products. Results of dry separation from the ball-milled coal are shown in table 9 and figure 14. About 50 pct of the pyritic sulfur, which amounted to about 70 pct of the available pyritic sulfur, was removed in rejects of 10 to 15 pct. The corresponding results with the Hardgrove-milled products (table 10 and fig. 15) showed about 35 pct pyritic sulfur removal, which amounted to about 50 pct of the available pyritic sulfur. Stage grinding of this coal improved the dry separation considerably, although the reject portion was higher. The results in table 11 and figure 16 show that about 65 pct of the pyritic sulfur was removed after three 15-min stages and one 30-min grinding stage. This pyritic sulfur removal amounted to nearly 90 pct of the available pyritic sulfur.
Conclusions
Conventional wet-washing methods effectively remove liberated pyrite down to fine size; however, to liberate the fine-size pyrite, additional crushing of the coal below transportation size is necessary. If these fine-size coals are cleaned by wet methods, problems of coal drying and wet-waste disposal are introduced. The dry-removal process investigated offers an alternate method of cleaning fine coals.
The dry-separation process when integrated with the grinding operation was nearly as effective as the float-sink separation and, because of its potential advantages, may warrant further research on a larger scale.
