The Gunnison, Colorado, tailings provide an example of the chemical reactions across the interface with soils rich in pyrite but low in calcite. In this case, the retardation of trace components is determined primarily from the Eh controls and less from the pH (Johnson, 1985). The compositions of groundwater from wells in the vicinity of the tailings were used to determine the reactions that occur between the tailings seepage and the groundwater/soil.
The water samples from wells around the Gunnison tailings showed four types of chemically distinct groundwater corresponding to different locations in the aquifer (Table 3). Type 1 groundwaters are from wells upgradient of the tailings and have a near-neutral pH range, a wide range in iron concentrations, and low concentrations of uranium and sulfate. Type 2 groundwaters are from monitoring wells in the upper 15 feet of the aquifer directly downgradient of the tailings. These waters have a pH slightly more acidic than the background waters, a wide range of iron concentrations, and consistently high concentrations of uranium and sulfate. Type 3 groundwaters are from wells in the same location as Type 2 waters, but at a depth of about 45 feet. The uranium and iron concentrations in the lower aquifer are distinctly less
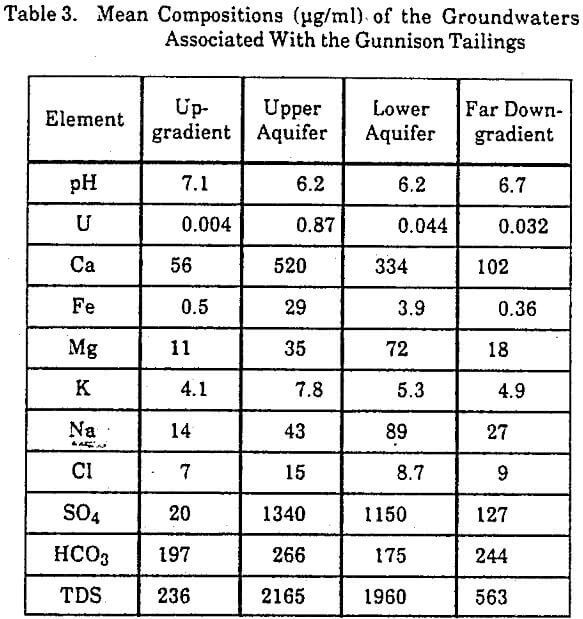
than in the upper aquifer, while the sulfate concentrations and pH are not significantly different in the upper and lower parts of the aquifer. Type 4 groundwaters are primarily from domestic wells farther downgradient than the Type 2 and 3 groundwaters. The chemical character of these waters shows influence from the tailings, although the acidity and concentrations of uranium, iron, and sulfate are less than in the waters nearer the tailings.
Measurements of Eh in the groundwater samples at the time of collection were attempted. Values obtained were within the range of +165 to -145 mVolts. Despite the variation and uncertainty in the measurement of Eh, the measurements suggest chemically reducing conditions. The odor of hydrogen sulfide gas in many of the wells further attests to reducing conditions as highly oxidized seepage from the tailings is introduced. The quantity of pyrite in the soils determines the buffering capacity of groundwater to maintain the reducing Eh conditions as highly oxidized seepage from the tailings is introduced. The oxidation of pyrite, coupled with the reduction of uranium, followed by the precipitation of uraninite and iron hydroxide, likely accounts for the decrease in soluble iron and uranium in the lower aquifer and downgradient groundwater (Equations 4,5, and 6).
UO2+2 + 2e- = UO2………………………………………………………………………(4)
2FeS2 + 2H2O = 2Fe+3 + 4SO4-² + 2e- + 4H +………………………………(5)
Fe+3 + 3H2O = Fe(OH)3 + 3H+……………………………………………………(6)
The groundwater compositions indicate that the acidity and concentrations of elements decrease along the flow path from the tailings through the aquifer. Modeling of the chemical reactions along the flow path can be considered as two sets of reactions: the reactions accounting for the changes at the interface between the water in the tailings and in the upper aquifer, and for the changes within the aquifer. Even though, conceptually, the reactions can be thought to occur in discrete zones of chemical equilibrium, the continuum of the reactions, with respect to progress toward chemical reaction and with distance along the flow path, must be recognized.
The equilibrium state of an average water from the four groundwater types was calculated by PHREEQE. The saturation indexes of water with respect to the minerals, calcite, gypsum, and uraninite (UO2), are given in Table 4. Uraninite was the most stable uranium mineral in the system. PHREEQE calculated the Eh of the solutions in equilibrium with the measured soluble iron concentrations and finely crystalline iron hydroxide.
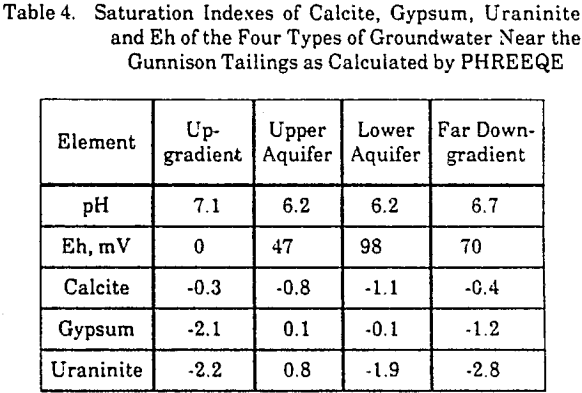
The saturation indexes of calcite, -0.3 and -0.4, in the background groundwaters and in those far downgradient from the tailings appear to represent a steady state of calcite in the groundwater mineral system specific to this site. The increase in degree of undersaturation with respect to calcite in the aquifer directly downgradient from the tailings suggests the consumption of calcite. Furthermore, it suggests that the quantity of calcite is not sufficient to buffer the groundwater downgradient from the tailings at background pH conditions. The near-zero saturation indexes of gypsum suggest that the concentrations of calcium and sulfate are determined by gypsum equilibrium. The positive saturation index of uraninite predicts the precipitation of uraninite along the flow path away from the tailings.
Calculations of the percent dilution of the tailings solution with the groundwater in the upper aquifer on the basis of the concentrations of the conservative elements. Na, K, and Cl, indicate that the upper aquifer is comprised of about 10 percent tailings solution. The concentrations of sulfate, uranium, and iron are an order of magnitude less, and the calcium concentrations are an order of magnitude greater than would result from 10 percent mixing. Also, the pH is about two units greater than is expected from mixing 10 percent tailings with the groundwater. These calculations indicate that calcium is added and acid, sulfate, iron, and uranium are deducted from the system by chemical reactions.
The increases in pH and calcium concentration suggest neutralization of acid by dissolution of calcite. Uncontaminated near-surface soils in the area of the tailings contain about 1 percent calcite. The soils directly below the tailings are virtually depleted in calcite, which supports the model of neutralization of the acid from the tailings by dissolution of calcite. After consumption of the calcite, the pH of the ground¬water will decrease if additional tailings seepage occurs. This situation accounts for the low pH of the groundwater directly below the tailings pile.
The lower concentrations of sulfate, iron, and uranium in the groundwater samples than would be expected from mixing of tailings solution with groundwater suggest precipitation reactions. The thermodynamic calculations by PHREEQE predict that the precipitating minerals are gypsum, iron hydroxide, and uraninite. The equilibrium of uraninite and iron hydroxide is controlled by the Eh and pH conditions of the groundwaters.
The lower iron and uranium concentrations between the upper and lower parts of the aquifer indicate further precipitation of iron hydroxide and uraninite. The kinetics of redox reactions are generally slower than simple dissolution/precipitation reactions. This may account for the apparent supersaturation of uraninite in the groundwaters in the upper aquifer. The uranium concentration in the wells farther down- gradient are greater than would result from dilution. This may indicate remobilization of uraninite as the Eh and pH conditions change, which increases the degree of under- saturation of uraninite.
Comparison of the saturation indexes of calcite and gypsum in the groundwaters from the lower aquifer and from farther downgradient shows that dilution reduces the sulfate concentrations to below gypsum saturation. The near background saturation index of calcite and the increase in pH indicate further neutralization of acid and suggest that there is probably calcite remaining in the solid phase which is buffering the groundwaters at the measured pH.
