The purest gold obtainable is required for use as standards or check pieces in the assay of gold bullion. The following method of preparing it is now in use at the Mint. Gold assay cornets from the purest gold which can be obtained are dissolved in nitrohydrochloric acid, and the excess of nitric acid expelled by evaporation with additional hydrochloric acid on a water bath. The blackish-red fused product, smelling of chlorine and consisting chiefly of AuCl3. HCl, or HAuCl4 (chlor-auric acid), is then poured in a thin stream into a large glass vessel full of distilled water, and a solution of about 1 oz. of gold in each pint of water (1 gramme of gold in 20 c.c. of water) is formed in this way. After vigorous stirring the solution is left to settle, and at the end of about a week, the whole of the precipitated chloride of silver will have subsided to the bottom. The progress of the subsidence is easily watched. The particles fall at the rate of about 3 or 4 inches per day. The clear bright supernatant liquor is now removed by a glass siphon, and diluted to about 1 oz. of gold per gallon of water (1 gramme to 150 c.c.). No further subsidence of silver chloride takes place, even if the diluted liquor is left 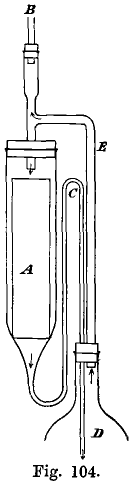 undisturbed for three months, and the gold may be at once precipitated. Oxalic acid is often used, and is stated by Kruss to be the best precipitant if platinum is present. If platinum is absent, as in good cornet gold, carefully purified sulphurous acid gas is a more convenient precipitant than oxalic acid, and may be substituted for it without any ill effects. Platinum is not precipitated from its solution as tetrachloride by sulphurous acid, and tellurium, though reduced by sulphurous acid at the same time as gold, would not be present in the solution under the conditions named. Sulphurous acid acts in a few minutes in cold solutions, but if oxalic acid is used, it is better to warm the solution and to leave it to stand for three or four days.
undisturbed for three months, and the gold may be at once precipitated. Oxalic acid is often used, and is stated by Kruss to be the best precipitant if platinum is present. If platinum is absent, as in good cornet gold, carefully purified sulphurous acid gas is a more convenient precipitant than oxalic acid, and may be substituted for it without any ill effects. Platinum is not precipitated from its solution as tetrachloride by sulphurous acid, and tellurium, though reduced by sulphurous acid at the same time as gold, would not be present in the solution under the conditions named. Sulphurous acid acts in a few minutes in cold solutions, but if oxalic acid is used, it is better to warm the solution and to leave it to stand for three or four days.
After the precipitated gold has settled, the acid solution is siphoned off and the gold transferred to a large flask and repeatedly shaken with cold distilled water, closing the mouth of the flask with a watch-glass. The gold is then boiled for a few days in an apparatus resembling Soxhlet’s fat separator. The gold is wrapped in well-washed muslin, and placed in the glass tube, A (Fig. 104). Water distills from the vessel, D, and the steam passes through the tube, E, and condenses in a condenser, placed above B, and drips on the gold. The water collects in A, and siphons over into D through the tube, C. The use of this washer was suggested by J. Phelps.
The gold is then turned out into a porcelain basin, covered over, dried, melted in a clay pot, and poured in an iron mould, which is neither smoked nor oiled, but rubbed with powdered graphite, and then brushed clean with a stiff brush. The ingot is cleaned by brushing and heating in hydrochloric acid, dried, and rolled out. The rolls must be clean and bright, and free from grease. The surface of the rolled gold plate is, again cleaned by scrubbing with fine sand and ammonia and also with hydrochloric acid, and is scraped with a clean knife before being used as proofs in bullion assay.
The amount of gold prepared at one time is usually from 40 to 50 ozs., but only 20 ozs. can be washed in a single flask of a capacity of 160 ozs. or 4½ litres, and only about 10 ozs. treated at one time in the Soxhlet apparatus. The addition of hydrobromic acid to the clear solution of gold chloride has been tried without throwing more silver out of solution or affecting the quality of the gold. An additional precaution is to remove the gases taken up by the gold during the process of melting by heating it to redness in vacuo, but the consequent improvement in fineness, if any, cannot be detected by assay.
