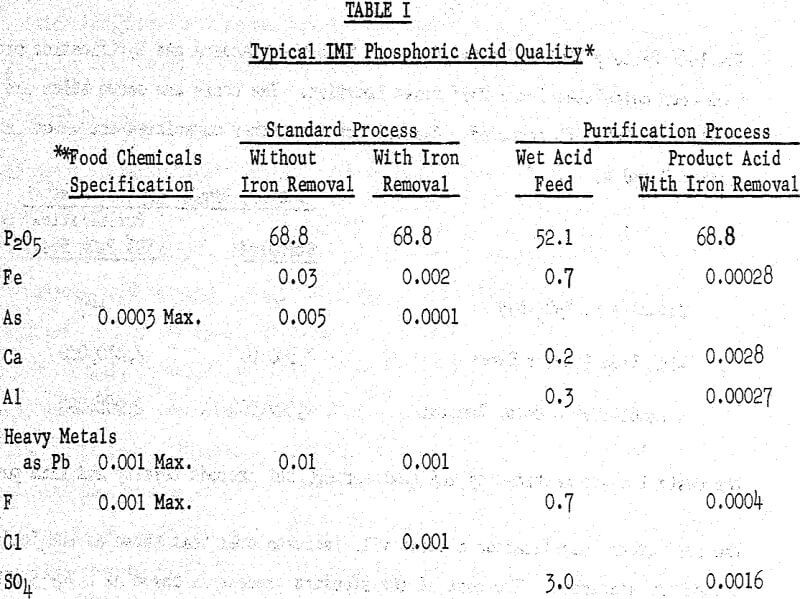
The Israel Mining Industries Institute for Research and! Development has developed, during recent years, a number of new processes for fertilizer materials. Their phosphoric acid processes, however, are capable of producing an acid of far higher quality than that normally associated with fertilizer grade acid.
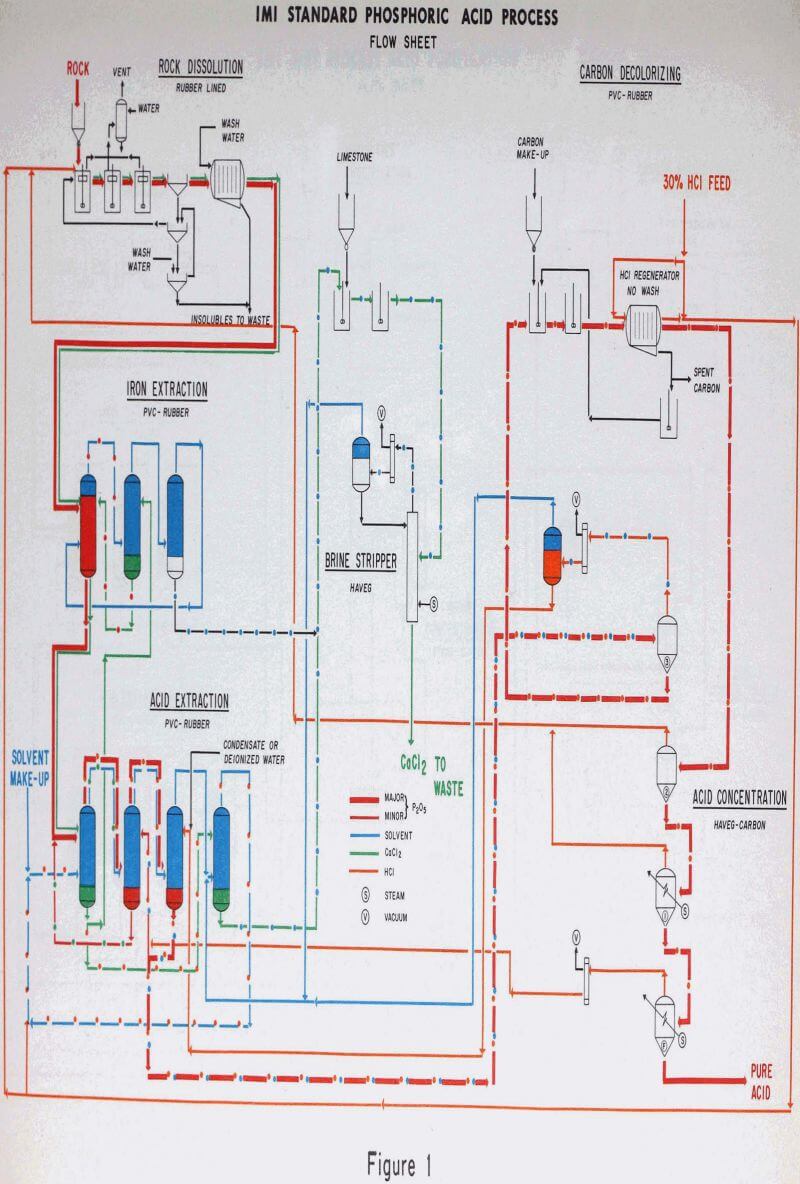
Unground phosphate rock is fed into the first of a series of three dissolvers along with recycle HCl, a portion of the HCl feed, and CCD wash water. The following are the major reactions in the acidulation of phosphate rock with HCl:
20 HCl + Ca10(PO4)6 F2 → 6H3PO4 + 10 CaCl2 + 2HF
6 HF + SiO2 → H2SiF6 + 2H2O
CaCO3 + 2HCl → CaCl2 + H2O + CO2
Fe2O3 + 6HCl → 2FeCl3 + 3H2O
Al2O3 + 6HCl → 2AlCl3 + 3H2O
Rock dissolution is quite rapid and is essentially completed in the second of the three dissolvers. There is considerable foaming which is controlled by a combination of a foam breaker paddle in the top of each dissolver and spraying solvent saturated water on the foam surface. The third dissolver serves to break any residual foam before the slurry goes to the thickener.
The polished dissolution liquor will contain, in addition to the phosphoric acid and excess HCl, essentially all of the calcium and most of the metallic impurities originally present in the rock. The key feature of this process is the solvent extraction system which separates the phosphoric and hydrochloric acids in this complex solution in a pure form, while rejecting the impurities to waste.
The dissolution liquor from the polishing filter is contacted with a relatively small quantity of solvent in the first extractor, extracting essentially all of the iron into the solvent phase. A small quantity of phosphoric and hydrochloric acids are co-extracted.
The dilute phosphoric acid, hydrochloric acid stream from the acid extraction section flows to the acid concentrator. The concentrator serves the simultaneous functions of solvent recovery, HCl recovery and phosphoric acid concentration.
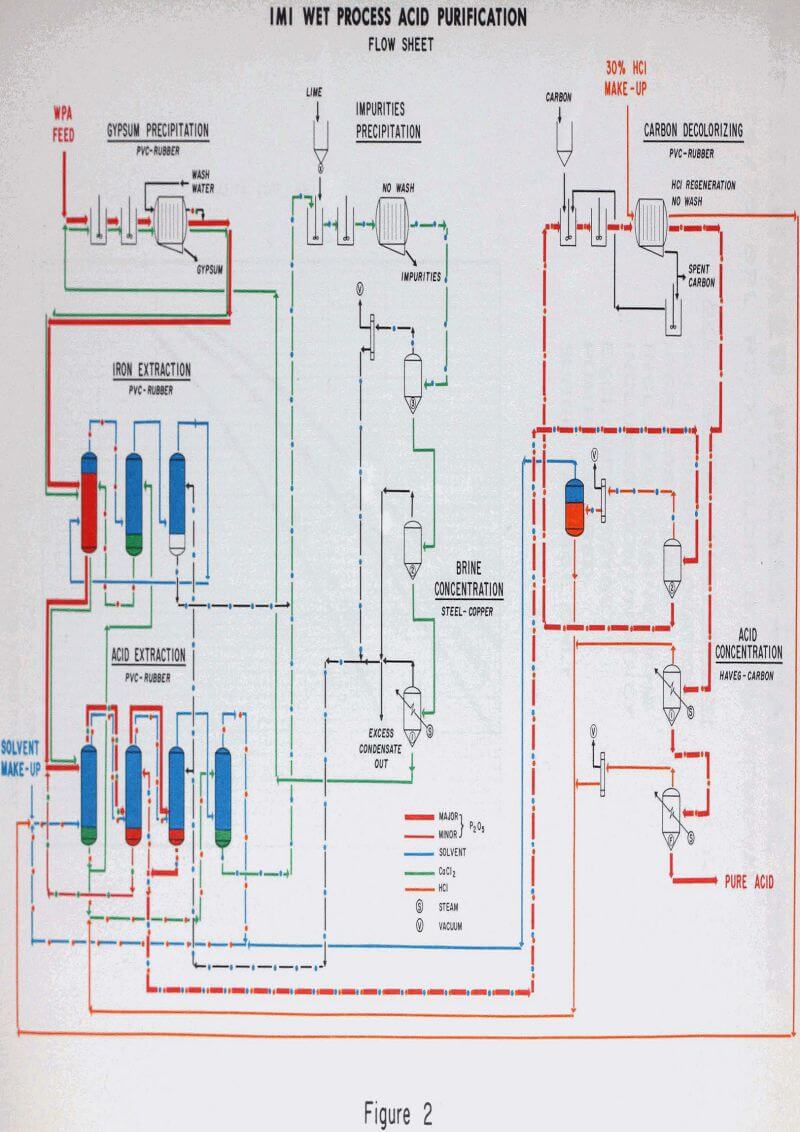
Wet process acid, either filter or concentrated acid, is mixed with a recycle purified CaCl2 brine. Gypsum equivalent to the sulfate content of the feed is pre¬cipitated according to the following reactions:
H2SO4 + CaCl2 + 2H2O → CaSO4·2H2O↓+ 2HCl
Fe2(SO4)+3CaCl2 + 6H2O → 3CaSO4.2H2O↓+ 2FeCl3
The approximately 3% solids slurry is filtered on a pressure filter and the gypsum cake is wasted. The acid CaCl2 filtrate is sent to the extraction section.
The CaCl2 brine from the phosphoric extractor and the aqueous iron stream from the iron extraction section are combined and neutralized with lime to precipitate all of the impurities removed from the acid feed. The dilute slurry is filtered in a pressure filter and the cake is discarded. The purified dilute brine filtrate is concentrated in a triple effect evaporator before being recycled back to the feed preparation section.
One of the expensive operations in the purification process is removal of impurities and concentration of the circulating calcium chloride stream. This operation can be avoided by HCl dissolution of rock equivalent to part of the P2O5 capacity required.