Table of Contents
The slime pulp or slurry on leaving the sand classifier with its proper admixture of lime is first led to some form of thickening device if, as is usually the case, the ratio of liquid to solid is too large for economic handling in the cyanide treatment. The form of thickener almost universally used in the early days consisted of a large spitzkasten composed of a number of pointed boxes arranged as a unit in such a way as to give a continuous superficial area.
At the Minas Prietas plant of Chas. Butters and Co., capable of treating 9000 tons of mixed sand and slime tailings per month, the slime spitzkasten was 36 ft. by 42 ft. composed of 42 pointed boxes 6 ft. square and 4′ 9″ deep; at the apex of each box was a nipple and hose discharging into a common launder.
 Fig. 11 The Dorr Thickener
Fig. 11 The Dorr Thickener
The slime pulp entered by a launder stretching from end to end along the centre line with small holes bored at intervals for the exit of the pulp, and the clear solution overflowed a weir at both sides, so that on each side of the feed launder there were three rows of seven boxes for the settlement of the slime. The overflow weirs were raised sufficiently to submerge the partition walls of the various compartments to a depth of nine inches, giving a continuous surface area of solution of 1512 sq. ft. and a depth of 5′ 6″ from the apex of each compartment to the surface of the solution. The apex discharges of the two rows nearest the intake were allowed to flow continuously, the discharges of the second rows were closed off part of the time and the third rows were only opened for a few hours each day, the general regulation being so arranged as to give as thick a Slurry as possible in the underflow while maintaining a clear overflow for return to the mill (or in this particular instance, to the pulping tanks).
This thickened pulp was at times further dewatered in a collecting tank, usually a large circular tank with peripheral overflow and fitted with mechanical stirring arms. The Slurry enters at the centre and when the tank is full the slime settles and a clear liquor overflows into the ring-launder to be returned to the mill. The pulp continues to flow in until the tank contains sufficient slime to constitute a treatment charge, when the stream is diverted to another collecting tank.
A very efficient modification of this type of collecting tank is the Dorr thickener. The entry of pulp and overflow of clear liquor are the same but in the Dorr system the thickened product is continuously removed from the bottom by means of slowly revolving rabbles which convey it to the centre where it falls into a discharge pipe whose aperture can be adjusted to regulate the speed of outflow. The device works smoothly and continuously and has superseded all the older methods for thickening slime pulp.
“The capacity of Dorr thickeners has been found to be primarily a function of area, although the depth of the tank has an influence depending on the dilution of the feed and the dilution of the underflow desired. With a given area and depth and a very dilute feed and underflow, the capacity depends on the amount of liquid that can be clarified; i.e. additional solids, but no additional liquid, can be added to a tank already fed to capacity without overflowing slime. On the other hand, with a feed perhaps 8 of liquid to 1 of solids and a thick discharge of 2 to 1 or less, it will be found usually that additional liquid can be added to a thickener operating at capacity without overloading it, but any addition of solids will cause slime to overflow.”
In actual practice the area per ton of solids thickened per 24 hours averages about 4 sq. ft. for an ordinary siliceous ore slime, but may rise as high as 10 to 15 sq. ft. in the case of light argillaceous slime.
The subject of the rate of settlement of slimes and the calculation of the area and depth required has been developed in considerable detail in a paper by H. S. Coe and G. H. Clevenger. Their investigations show that slime Slurry in process of settling passes through two main phases, that of “free settling” and that of “compression,” the two zones being separated by a transition zone.
By the phase of “free settlement” is meant that condition of density in which the flocks or curds of colloidal matter that are produced by the electrolyte are sufficiently dispersed to settle by gravity unhindered through the liquid in which they are suspended, such settlement taking place at a constant or gradually diminishing rate until the critical point is reached, at which point the phase of compression begins. The “hindered settling” or compression phase is that in which the flocks have drawn so close together that no further settlement can take place without a compression of the flocks themselves, the liquid displaced in the process having to force its way out of the compression zone by forming channels therein leading up into the higher zones. It is thus apparent that in calculating the cubic capacity of tank necessary for any given slime these two separate factors have to be taken into account and separately determined.
In regard to the determination of the critical point dividing free settlement from compression, it is not always easy to reach a conclusion. Indications of the latter phase are a marked retardation in the settlement rate and the formation of channels of clear solution through the mass of solids. Referring to the characteristics of the pulp while still in the free settlement zone the authors of the paper referred to say “ordinarily the latter point is indicated by the evenly flocculated appearance of the Slurry surface, without channels of fluid coming through, and by the uniform texture of the pulp as seen in a glass cylinder.”
For determining the settling area necessary the Dorr Company gives the following outline instructions adapted by members of its staff from the paper referred to. It should be remembered, however, that the conditions of different ores are so varied that it is difficult to give a short and concise method of procedure applicable to all cases. Two rules should be carefully observed: one is that the sample should not be allowed to dry either by heat or by atmospheric evaporation between the time it is taken and the time the tests are made: the other is that to insure representative results it is not sufficient that the ore should be crushed to the desired fineness but that it should also be subjected to conditions of agitation similar as regards time and method to those which are to obtain on the milling scale.
“When a mixture of liquid and solids is fed to a Dorr Thickener at a certain dilution say 5:1 (liquid : solids) and the discharge at the bottom contains 50% moisture or a dilution of 1:1, the Slurry in settling from 5:1 to 1:1 passes through all the intermediate dilutions 4:1,3:1,2:1.
“At different depths in the Thickener there will be different dilutions or zones of pulp. One of these zones will have a smaller capacity and so require a larger settling area than any of the others and so the amount of solids fed to a given tank cannot exceed the capacity of that slowest zone and the area must not be smaller than the area necessary for the slowest zone.
“To test for the size of a Thickener unit then, pulps are made up of different dilutions between the dilution of feed and the dilution of discharge. These pulps are put in 1000 cc. graduates and shaken. The rate of settlement of each Slurry for 10 minutes is noted and these observations checked by shaking and settling again.
Having obtained these dilutions the area required per ton of solids, is calculated by the formula
A = 1.333 (F – D)/R
Where A is the area in sq. ft. per ton of solids per 24 hours, F is the ratio of solution: solids which settle at the rate of R feet per hour and D is the ratio of solution: solids in the discharge required from the Thickener. 1.333 is 2000 ÷ (62.5 X 24) which is a factor necessary in order to get the desired units.
“F and R are determined then for each graduate and the area required for each dilution calculated. The largest calculated area will be the unit area necessary and this multiplied by the tons of solids to be thickened per 24 hours will give the total settling area necessary.”
(1) Take 1000 cc of pulp feed to Thickener.
A. Put above in a 1000 cc graduate.
Shake.
Allow to stand and note readings between Slurry line and clear solution.
At end of shaking………………………………..1000 cc
After 2 minutes……………………………………….cc
After 4 minutes……………………………………….cc
After 6 minutes……………………………………….cc
After 8 minutes……………………………………….cc
B. Decant 150 cc of solution.
Shake.
At end of shaking…………………………………..850 cc
After 2 minutes………………………………………..cc
After 4 minutes………………………………………..cc
After 6 minutes………………………………………..cc
After 8 minutes……………………………………….cc
C. Decant 150 cc of solution.
Shake.
At end of shake……………………………………….700 cc
After 2 minutes………………………………………….cc
After 4 minutes………………………………………….cc
After 6 minutes………………………………………….cc
After 8 minutes………………………………………….cc
Weight of dry solids in graduate
Number of cc corresponding to 1 foot of length of graduate
(2) Take 1500 cc of pulp feed to Thickener.
Allow this to settle and decant 500 cc of clear solution. Put remainder in 1000 cc graduate.
Shake and on standing note readings below.
A. At end of shaking……………………………………1000 cc
After 2 minutes……………………………………………….cc
After 4 minutes……………………………………………….cc
After 6 minutes……………………………………………….cc
After 8 minutes……………………………………………….cc
B. Decant 150 cc.
At end of shaking…………………………………………….850 cc
After 2 minutes………………………………………………….cc
After 4 minutes………………………………………………….cc
After 6 minutes………………………………………………….cc
After 8 minutes………………………………………………….cc
C. Decant 100 cc.
At end of shaking……………………………………………….750 cc
After 2 minutes……………………………………………………cc
After 4 minutes……………………………………………………cc
After 6 minutes……………………………………………………cc
After 8 minutes……………………………………………………cc
Weight of dry solids in graduate
Number of cc of dry solids corresponding to 1 ft. of length in graduate
(3) Take 2000 cc of Slurry feed to Thickener.
Allow this to settle and decant off 1000 cc of solution. Put remainder in 1000 cc graduate.
Shake and on standing note readings below.
A. At end of shaking………………………………………1000 cc
After 2 minutes…………………………………………………..cc
After 4 minutes…………………………………………………..cc
After 6 minutes…………………………………………………..cc
After 8 minutes…………………………………………………..cc
B. Decant off 150 cc of solution Shake.
At end of shaking………………………………………………..850 cc
After 2 minutes………………………………………………………cc
After 4 minutes………………………………………………………cc
After 6 minutes………………………………………………………cc
After 8 minutes………………………………………………………cc
C. Decant off 100 cc of solution Shake.
At end of shaking…………………………………………………..750 cc
After 2 minutes………………………………………………………..cc
After 4 minutes………………………………………………………..cc
After 6 minutes………………………………………………………..cc
After 8 minutes………………………………………………………..cc
Weight of dry solids in graduate
Number of cc corresponding to 1 ft. of length of graduate
It will be noted that the successive decantations of clear portions of the solution are a convenient method for making the pulps of different densities.
The ratio of liquids to solids at each density is found by calculation from the specific gravity of the dry slime which must of course be determined. For instance, in the case of (1) A: assume that when the reading is taken the line of pulp has settled to 800 cc and that the weight of dry solids present found by subsequently drying the whole is 190 grams. Then if the specific gravity of the dry slime be taken at 2.6 and that of the liquid as 1 the ratio of liquid to solid in the 800 cc of Slurry in the cylinder will be found as follows: the 190 grams of slime being 2.6 times heavier than water will occupy the space of 190/2.6 = 73 cc of water in the cylinder: then 800 cc of water less 73 = 727 cc, so that at the 800 cc mark there are 727 grams of water and 190 grams of dry slime, the ratio of the two being 727:190: :3.8:1.
NOTE.—After each shaking it is well to allow the cylinder to stand for from three to five minutes before beginning to time the settlement so as to allow the colloids time to assume the flocculated condition which allows settlement to begin. It will often be found that if the timing is started immediately after shaking the settlement during the second five minutes will be considerably more rapid than during the first five minutes, the second reading being therefore more representative of working conditions.
The second or compression phase of settlement cannot be determined by this formula so it is very important that the foregoing tests should not be carried beyond the critical point or the end of the phase of free settling.
Determination of D (the ratio of solution to solids in the discharge required from the thickener). There is a point of maximum density beyond which any given slime will not settle and it is of course useless to require a discharge of greater density than this. In the Dorr Company’s instructions already quoted this point is determined as follows:
“The dilution of discharge “D” that can be expected may be determined by allowing a pulp of consistency about 3:1 to stand about 48 hours in a graduate stirring gently with a glass rod every four or five hours.” For instance,
“ Take 1000 cc of pulp at approximately 3:1.
Shake.
Allow to stand.
At end of shake………………………………………………………1000 cc
After ¼ hour…………………………………………………………….cc
After ½ hour…………………………………………………………….cc
After ¾ hour…………………………………………………………….cc
After 1 hour………………………………………………………………cc
After 1½ hours………………………………………………………….cc
After 2 hours…………………………………………………………….cc
After 4 hours…………………………………………………………….cc
After 8 hours…………………………………………………………….cc
After 12 hours……………………………………………………………cc
After 16 hours……………………………………………………………cc
After 20 hours…………………………………………………………..cc
After 24 hours…………………………………………………………..cc
After 36 hours…………………………………………………………..cc
After 48 hours………………………………………………………….cc
Note.—After each reading decant as much clear solution as possible.
Dry weight of solids in graduate.
Number of cc corresponding to 1 foot of length of graduate.
Then if the slime needs 36 hours to attain its maximum density or the density required, the cubic capacity of the thickener must be sufficiently large to retain any given portion of the Slurry in that tank for the period ascertained as necessary by the test.
The following example is given by Coe and Clevenger on a pulp that required 4.31 sq. ft. of area per ton of solids per 24 hours for free settlement. A compression test was made in a cylinder 12 in. deep with a 3 to 1 Slurry, giving these readings:
“ Since an area of 4.31 sq. ft. is required per ton of solids per 24 hours the total solids per square foot retained in the thickening zone must be 19 X 2000/24 X 4.31 = 367 lb. or 19 hours supply per square foot. There is required a 5-hour supply of each of the pulp consistencies, 1.16 to 1, 1.275 to 1, 1.47 to 1, and a 4-hour supply of a 1.7 to 1 pulp.
“The solids per cubic foot in the above pulps are 43.2 lb., 37.6 lb., 33.7 lb., and 30 lb., respectively. The depth of each class of pulp would therefore be 2.23 ft., 2.57 ft., 2.87 ft., and 2.58 ft., or a total depth of 10.25 ft. To this depth must be added a foot for the loss due to the pitch of the drags in the thickener and 1.5 ft. for the depth of the feed, since the feed is thick and the volume will be proportionately low. The total calculated depth of the tank would be 12.75 ft. If proper allowance were made for storage capacity, the tank might be inconveniently deep. In this case it would be better practice to make the tank 12 ft. deep and make various allowances for additional capacity by increasing the diameter of the tank to give a 30 per cent, increase in area.”
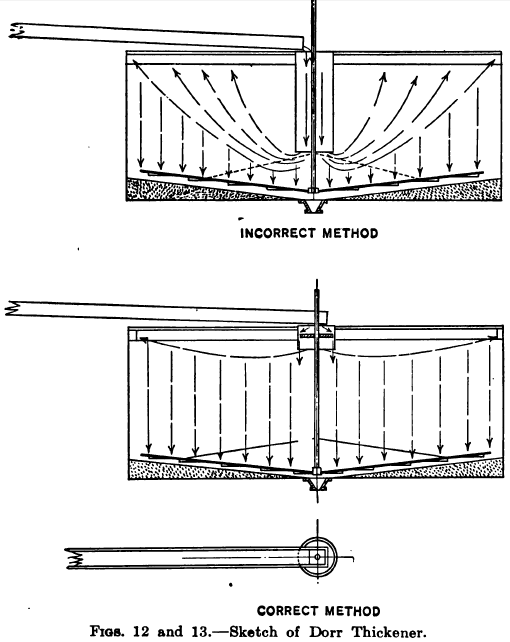
In regard to the practical operation of Dorr thickeners the waiter has observed in some instances a tendency on the part of the operator to extend the vertical intake cylinder down from 2/3 to ¾ of the depth of the tank, under the impression that the settlement would be helped thereby, the idea apparently being that if the slime is fed to the lower part of the tank it will stay there. This practice, however, defeats its own object and changes the apparatus from a settler into a classifier. The reason of this will be seen from the diagrammatic sketches, Figs. 12 and 13. Fig. 12 illustrating the incorrect arrangement shows how the deep cylinder destroys the tranquility of a large part of the tank charge causing upward currents which tend to raise and carry off the more flocculent material, and reducing the available settling capacity of the tank. The action may be compared with that of a spitzlutte, wherein the further the baffle-plate is lowered the greater is the velocity and lifting power of the current in the lower part of the box. Fig. 13 shows the more approved arrangement in which the intake cylinder extends down only from 12 in. to 18 in. below the surface or just sufficient to prevent the surface disturbance which would be caused by the inflowing pulp, such disturbance being further minimized by allowing the stream to fall onto a deflecting board, either floating on the surface or fixed close to the surface. This deflector is made of smaller diameter than the cylinder so as to allow an annular space for the Slurry to pass downward into the charge. It may also be made in the form of a shallow tray with holes bored all around the vertical sides, and this plan works well when a screen is used to intercept waste and other matter which would tend to block the holes.
Coagulation of the Slime for Settlement
Since the slime treatment is conducted at a much lower ratio of liquid to solid than that usually employed in the operations of milling and classification there must be a dewatering of the slime between the mill and the cyanide plant. Such dewatering is almost always affected by settlement and decantation but owing to the invariable presence in the pulp of amorphous or clayey matter, it is impossible to obtain a settlement accompanied by a clear effluent liquor without the presence of some substance which will cause a coagulation or flocculation of this clayey material. Such substances or the solutions resulting from them are known as electrolytes, in view of the electrical nature of the phenomena exhibited by finely divided matter in suspension.
Role of Electrolytes in Cyanide Leaching
The substance most commonly used for this purpose is lime both on account of its cheapness and also because of its usefulness as an alkaline protector for the cyanide. Many other substances have a similar effect though varying in degree, such as alum, calcium carbonate, sulphuric acid, ferrous and ferric sulphate, and calcium sulphate. Lime usually forms a coarser grained and more flocculent curd with a more brilliantly clear supernatant liquor than any other electrolyte, but the precipitated mass does not settle as densely as that produced by some of the others. Caustic soda in some cases acts as a coagulant and while the liquor produced is not quite as clear as that resulting from lime, the curd has a finer grain and settles more slowly but much more densely. Sodium carbonate, on the other hand, tends to prevent coagulation and renders clarification almost impossible.
Effect of Lime Addition on Thickener Settling Rate
When milling is done in cyanide solution the whole of the lime necessary is usually added to the ore before it enters the mill; by this means it gets pulverized with the ore and performs its function of protective alkali. Milling in water is (1919) seldom employed except when it is desired to pass the Slurry over amalgamated plates before cyanidation. Even in these circumstances the lime is often added in the mill, notably in the Rand practice. When the method was first adopted there it was strongly objected to by the mill men on the ground that it hardened the amalgam. As soon, however, as they got accustomed to the changed conditions, it was found that no detrimental results followed; in fact, in some cases a marked increase occurred in the amount of gold recovered on the plates due to an improved amalgamation of the fine gold in the slime. As a consequence certain slimes which it had been designed to treat by cyanide were now found to be low enough in gold content to be run to waste. In other parts of the world the plan of amalgamating in lime water has not commended itself after fair trial and the necessary quantity of lime is added (usually in the form of milk of lime) to the ground ore after amalgamation, or even later on, as at the Homestake, after the separation of sand and slime.
The amount of lime added varies of course with the ore, but for satisfactory settlement of the slime a strength in the solution of 0.02% CaO is usually sufficient. When milling in cyanide the quantity of lime maintained in solution is regulated rather with a view to protective alkalinity and is usually considerably in excess of the bare amount needed for slime settlement.
