Table of Contents
The problem of producing a uniform, medium-fine sand for glass-furnace feed has been of interest to the glass-container industry for many years. In the present investigation of the problem, conducted by the Bureau of Mines in cooperation with the Owens-Illinois Glass Co., Alton, Ill., a satisfactory method of producing such sand was developed. The investigation involved continuous-grinding studies of glass sand with flint and porcelain pebbles, steel balls and rods, and a simultaneous study of classification.
The most satisfactory procedure consisted of grinding in a pebble or ball mill followed by a double classification: hydraulic classification to separate the oversize for return to the grinding unit and mechanical classification to remove fines and impurities from the ground sand. The proper application of this flowsheet usually yielded a graded sand containing less iron than the feed even when grinding was done in a steel-lined ball mill.
Background
The advantages in utilizing a uniform, medium-fine sand as a feed for glass furnaces has motivated a considerable amount of research on the problem of producing such sand by glass producers and others. For the glass producer, the faster melting rate of such a graded sand offers the possibilities of reduced fuel costs and increased furnace capacity. In addition, there is the likelihood of improving the quality of the melt and thereby reducing the number of seeds (air bubbles) and stones (unmelted grains) in the finished ware.
Although the melting rate of a glass batch depends on other factors in addition to the grain size of the sand, there is experimental evidence to show that grain size influences the melting rate considerably. Potts, Brookover, and Burch found that when the raw materials (sand, soda ash, and limestone) were always matched in size, there was a great decrease in the melting time as the grain size of the materials was decreased. They found, too, that when the grain sizes of the soda ash and limestone were held constant at 40 to 60 mesh, the minimum melting time occurred when 40 to 60 mesh sand was used. The increase in melting time with decrease in grain size beyond 40 to 60 mesh was due, they postulated; to accelerated demixing or separation of batch during melting as the grain size of the sand decreased in relation to the grain size of the other materials.
Sand-preparation Practice
Although the American Ceramic Society and the National Bureau of Standards have established a recommended grading for glass sand, manufacturers have not adhered to the recommendation. The practice in glass-sand preparation is directed, for the most part, to recovering the sand grains at the natural size in which they occur in the sandstone. Although the flowsheet may vary in different plants, the process is essentially one of liberating the individual grains in the sandstone, washing to remove excess fines and impurities and screening on about 20 mesh to separate any oversize. This is particularly true of most of the operations in the loosely cemented St. Peter sandstone of northern Illinois and Missouri.
In other areas, where more firmly consolidated sandstones are found, more rigorous treatment is required to liberate the individual sand grains. The operations in the Pennsylvania and West Virginia fields have standardized on the chaser mill or wet-pan grinder as a fine crusher before washing. In a very few installations, a tube or ball mill has been used to supplement the action of jaw crushers and washers in disintegrating compact sandstones. In none of these installations, however, is the grinding unit used to prepare a specification sand for the glass industry. Whatever comminution takes place is incidental to the objective of freeing the individual grains in the sandstone.
Present Investigation
Description of Sample: The present investigation was made on a 35-ton sample of minus 20 mesh
washed silica sand from the Ottawa Silica Co., Ottawa, Ill. The sample was essentially silica with small amounts of pyrite, rutile, tourmaline, iron oxides, and other heavy minerals. Some of the silica grains contained inclusions of the accessory minerals; others had surface stains of iron oxide. Chemical analysis showed 0.024 to 0.033 pct total iron reported as ferric oxide. The average sieve analysis of more than 25 samples of the feed sand collected during this investigation is shown in table III.
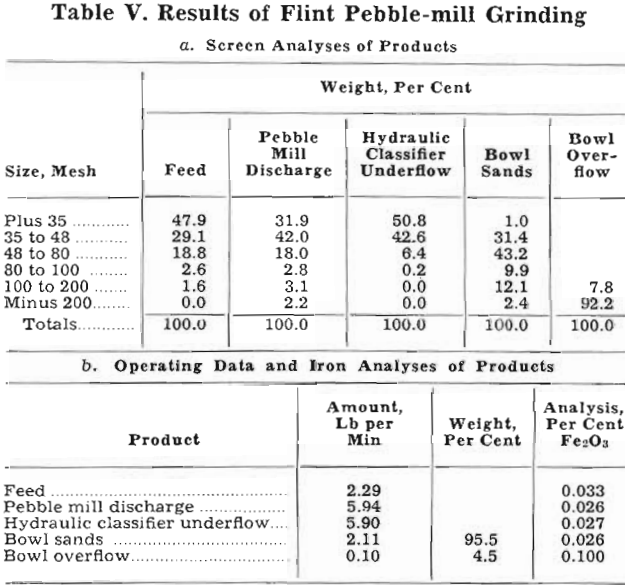
Grinding with Pebble Mill: To avoid contamination of the sand by iron, the first series of experiments was made with a pebble-mill grinding circuit. The mill, 15 by 36 in., was lined with silex blocks and charged with 145 lb of 1 to 2½-in. porcelain pebbles. The ends were lined with rubber belting, and the mill had a 6-in. discharge. Other items in the experimental setup included a single- spigot, hydraulic classifier, a 3-ft bowl classifier, and necessary sand pumps. In addition, an 8-in. spiral was utilized to dewater the underflow from the hydraulic classifier and convey it back to the pebble mill. A photograph of the entire unit is shown in fig. 1.
The hydraulic classifier was the subject of considerable experimentation before a satisfactorily operating device was constructed. It was patterned after an open-bottom hydraulic classifier which was developed in another Bureau of Mines laboratory and described in another publication. The fact that the present problem was concerned with the classification of a single mineral permitted some simplification. It was constructed of a piece of 4-in. pipe 26-in. long fitted with a conical bottom. Feed was introduced at a point 6 in. from the top, and the hydraulic water entered 9 in. from the bottom. Fig. 2 is a photograph of the classifier. The machine was fitted with a periodic pincers which has been used on other materials to give a semicontinuous discharge of spigot product. In this case, however, it was unsatisfactory; repeated opening and closing of the pincers caused such surging of the sand pulp that classification was impossible. A few tests soon showed the most satisfactory continuous spigot discharge was through a soft rubber hose constricted by a screw clamp.
Grinding with Rod Mill: The next phase of the investigation was a brief study of grinding the sand in a rod mill. The rod-mill shell had the same external dimensions as the pebble mill so that the two could be used interchangeably on the same driving mechanism. The rod-mill shell was lined with Ni-Hard liners, and the ends were lined with ½-in. rubber belting so that the internal dimensions of the mill were 16½ by 35 in. The mill had a 12-in. discharge to facilitate rapid exit of the ground pulp and was charged with 300 lb of rods. The rods were ¾ and 1-in. SAE 4150 steel, which had been hardened by heat treating and oil quenching to give a surface hardness of 45 Rockwell C. Fig. 3 is a close-up showing both the rod and pebble mills.
Historical concepts of the economics of the glass-sand industry are changing rapidly. The greatly expanded demand for glass containers combined with higher freight rates on raw materials and manufactured products have induced a migration of glass factories toward densely populated centers and the creation of new standards of place value for sub-standard sand deposits. This migration has been facilitated by the construction of pipelines to bring cheaper natural gas and liquid fuel to large cities and may be further speeded by the adoption of modern mineral dressing methods to permit economical utilization of local raw materials.
Ordinarily the most objectionable, as well as the commonest, impurity from the standpoint of the manufacturer of any but the cheaper qualities of colored glass is iron. No natural sands are really pure silica. Even water-clear quartz crystals are likely to contain impurities in the form of solid solution as well as inclusions which cannot be eliminated except by chemical treatment that will break down the silica lattice. Pure white pegmatite quartz will usually analyze 0.01 pct or more of iron oxide, and ordinary vein quartz and the quartz grains in most crystalline rocks may be quite impure. Commercial glass sand is almost always won from sedimentary deposits, including unconsolidated beds and lightly-cemented, friable sandstones.
The Mineral Dressing Problem
Given a representative sample of a deposit, the first step in determining its amenability to commercial methods of purification is a petrographic study. Ordinarily, this calls for a screen analysis, each size fraction then being examined by microscopic grain counts, supplemented when necessary by heavy-liquid, high-intensity magnet, and other tests. The object of this preliminary examination is to identify all the minerals and to determine whether the iron occurs principally or significantly in one or more of the following ways:
- In clay nodules, ferruginous clay bond, or kaolinized feldspar soft enough to be dispersed in water and eliminated by light scrubbing and water washing.
- As adherent crusts or stains on the surfaces of the quartz grains.
- As disseminated or penetrated particles in the quartz grains.
- As discrete particles of limonite, magnetite, ilmenite, pyrite or other heavy iron minerals.
- As discrete particles of minerals containing iron as an accessory constituent, such as hornblende, garnet, glauconite, or biotite.
- As an impurity (solid solution?) in discrete particles of muscovite, sericite, rutile or zircon.
- As adherent iron-oxide crusts or stains which alter (activate) the surface characteristics of heavy mineral grains.
- Mechanically included in some lighter mineral such as cracked or weathered feldspar in arkosic sands.
Methods for removing soft clay are too well known to require discussion in this paper. They are common to ordinary sand and gravel preparation and correspond to desliming of ore pulps.
Discrete particles of heavy minerals are almost invariably smaller than the average quartz particles and thus are almost ideally prepared for easy removal by gravity separation. Tabling is the time-honored method but the Humphreys spiral and other devices should also be tried.
The characteristically small size of the heavy mineral particles is also a favorable factor in froth flotation. Flotation likewise may be effective in removing locked, iron-encrusted, or heavily stained particles of quartz and other minerals regardless of their apparent specific gravity.
Chemical Treatments
Acid leaching has been used with technical success with mineral acids alone or in combination with various reducing agents. It has generally been considered expensive, but under the conditions outlined at the beginning of this paper, it may not be too expensive where cheaper methods fail. Although this method may be effective in dissolving small amounts of finely-divided discrete particles of iron and titanium minerals, its principal use is for removing limonite specks and stains. When clay or other impurities are present, preliminary scrubbing and desliming are usually indicated.
Attrition Scrubbing and Grinding
The attrition scrubber devised by the Bureau of Mines at College Park, Maryland for preparing clean surfaces for froth flotation may be used to rub off the coating on mineral grains without breaking the grains. It is essentially a drastic blunging device with a high-speed rotor and stationary baffles. One model has a vertical shaft carrying radial spokes or blades; another has vertical blades, a design inspired by the Fagergren flotation cell.

