Average composition of ore from the San Bernardino deposit is less than 0.1 pct ThO2, 25 to 35 pct calcite, 10 pct rare earth oxides, 15 to 20 pct silica, and 30 to 40 pct barite. Rare earth content of the ore analyzes 50.7 pct CeO, 4.2 pct Pr6O11 11.7 pct Nd2O3, 1.3 pct Sm2O3, and 34.3 pct La2O3 and others.
By floating the barite and depressing the bastnasite, Molybdenum Corp. of America produced a concentrate that contained 60 to 70 pct rare earth oxides. More than 25 pct of the rare earths, however, was lost in flotation. It seemed likely that a more economical method could be found.
At the present time most of the rare earths in the concentrates are converted to rare earth chlorides, which are either fed to ion exchange columns for separation of the individual rare earths or reduced to misch metal. Current investigation shows that solvent extraction may achieve this separation more economically than ion exchange.
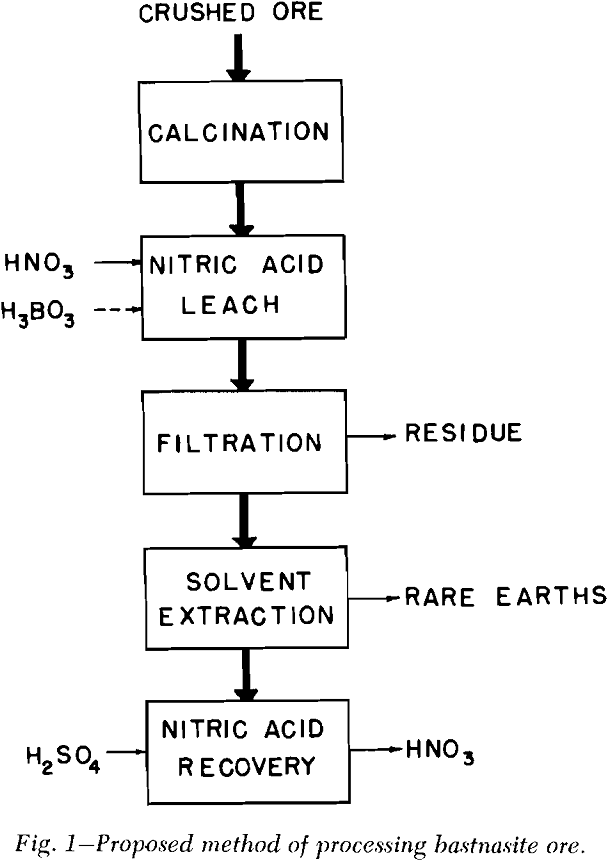
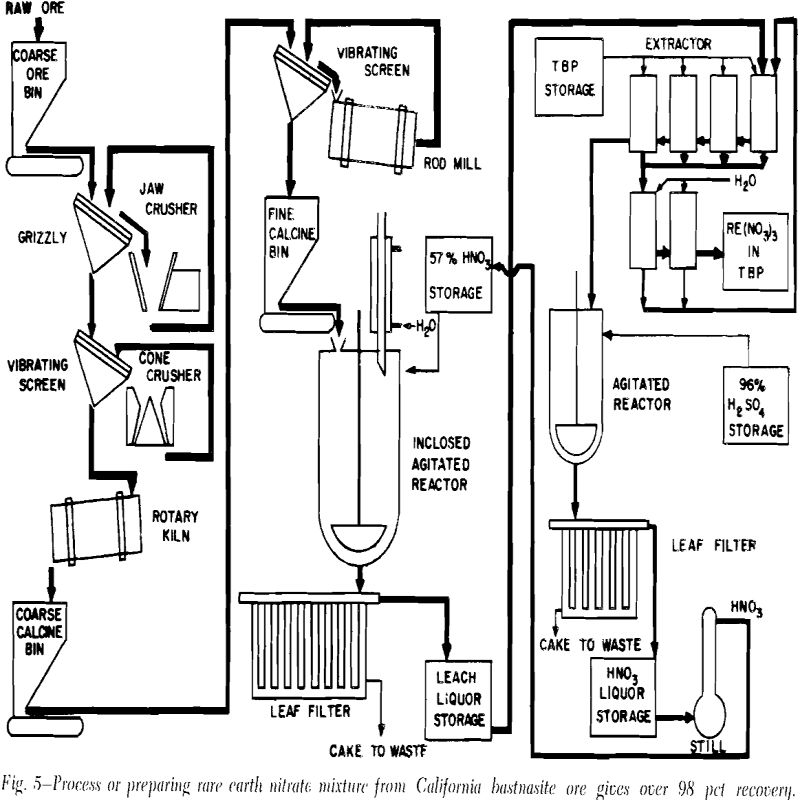
Calcination: Since California bastnasite ore contains 25 to 35 pct calcite and about 10 pct bastnasite, calcination was needed to reduce the violence of
reaction with nitric acid. Weight losses of 26 to 30 pct were obtained by calcining the ore at 900°C for 1 hr or at 800°C for 4 hr.
Leaching the Calcine: When the bastnasite calcine was leached with 30 pet nitric acid for 8 to 10 days, rare earth recoveries of only 45 to 50 pct were obtained. Because of the low rare earth recoveries, another method of opening the ore was sought.
Additional tests showed that leaching the calcine for only 1 hr liberated over 93 pct of the rare earths. The percentage recovered was not appreciably affected by increasing the leach time to 2 hr or more. These experiments also revealed that size of calcine particles had very little effect on the percentage of rare earths recovered.
Filtration Studies: It is difficult to predict accurately, from small-scale laboratory tests, the filter area needed in commercial plants. Several standard tests have been devised, however, from which it is possible to predict semi-quantitatively the requirements for a large plant.
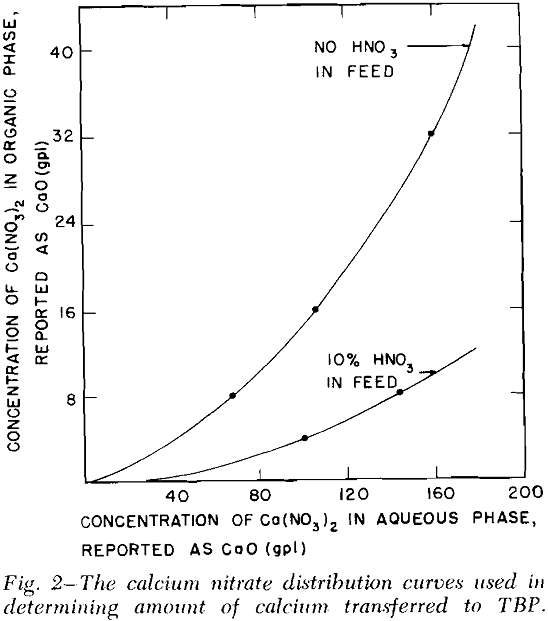
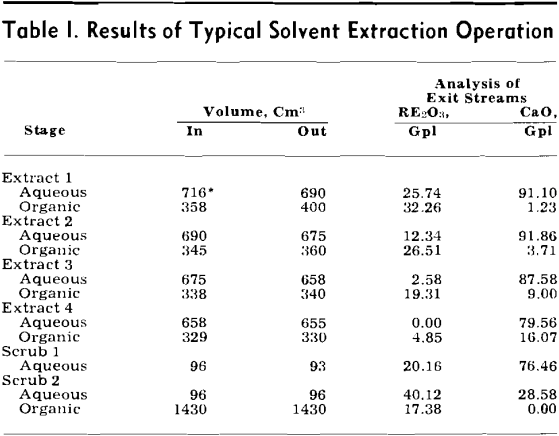
Solvent Extraction: Solvent extraction should be one of the cheapest and simplest methods of removing the combined rare earth nitrates from the leach liquor. According to Knapp,’ the distribution coefficient of the combined rare earths was greatly increased by the presence of salting agents such as calcium and ferric nitrates. The distribution curves for the combined rare earth nitrates were obtained by three to six successive contacts of the leach liquor with tributyl phosphate (TBP) which contained no diluent.
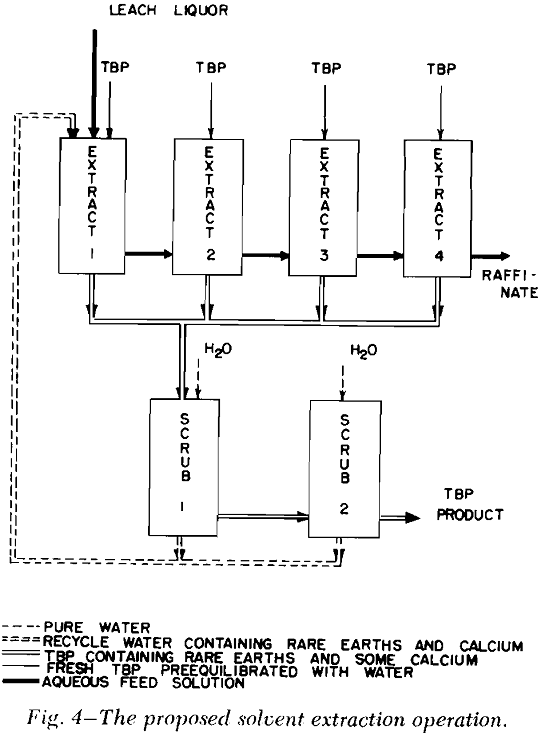
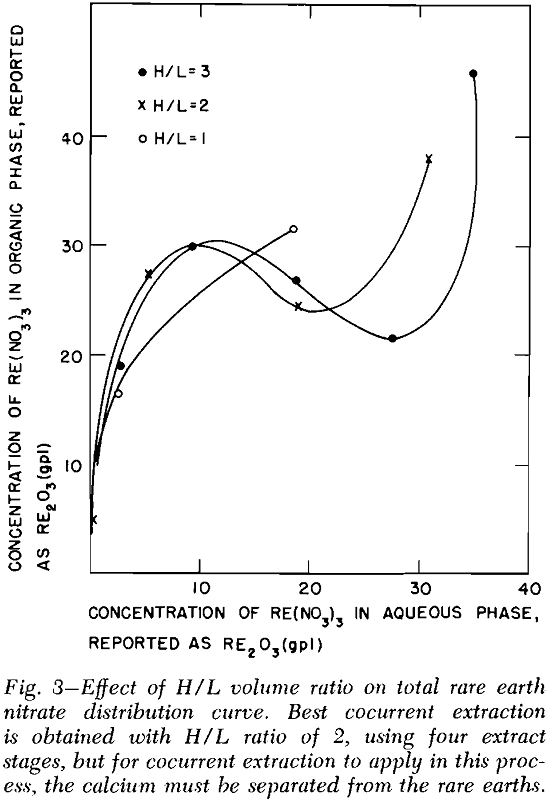
Bench-Scale Investigations
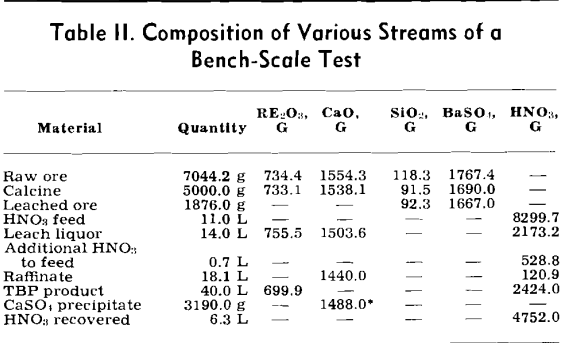
About 7200 g of raw ore were thoroughly mixed, sampled, and calcined. After the calcine had cooled, it was crushed to pass a 10-mesh screen, remixed, and sampled. Five kilograms of the calcine were slowly fed to 11,000 ml of about 57 pct nitric acid. The slurry was thoroughly agitated throughout the leaching operation.
Any calcium nitrate that crystallized upon cooling was dissolved by diluting the leach liquor with water. A small portion of the leach liquor was then used in the solvent extraction operation.
A fraction of the raffinate from the solvent extraction operation was processed to recover the nitric acid that had combined with the calcium and other impurities. Over 92 pet of the nitric acid in the raffinate was recovered in this operation. Undoubtedly greater nitric acid recoveries can be obtained; however, the optimum conditions could be more easily determined in a pilot plant operation.

