Table of Contents
- Current Chromium Recycling Methods
- Treatment of Superalloy Scrap to Recover Individual Elements
- Experimental Work
- Superalloy Scrap
- Composition of Superalloy Scrap
- Form of Superalloy Scrap
- Melting and Casting
- Oxidation Tests
- Quantity of Oxygen Required
- Rate of Deoxidation
- Character and Composition of Slag
- Recovery of Metals
- Effect of Cooling Rate on Element Distribution
- Integrated Oxidation-Sulfidation Tests
- Melting
- Sulfidation
- Casting
- Granulation
- Refractory Wear Tests
- Furnace Emissions
- Separation of Sulfides
- Chromium Scrap Flotation
- Determination of Optimum Grinding Time
- Effect on Magnetic Separation
- Effect on Flotation Separation
- Chromium Magnetic Separation Conditions
- Flotation Separation of Nickel and Chromium Sulfides
- Effect of pH
- Effect of Collector
- Mineralogical Studies
Described here is a process for recovering chromium and other metals from superalloy scrap. Laboratory-scale experiments were conducted to test a complex flowsheet utilizing a wide range of extractive metallurgical operations. The novel basis for the process is the formation of a sulfide matte in which chromium is concentrated in a discrete chromium sulfide phase. Mineral processing and hydrometallurgical procedures are used to separate chromium sulfide from the other matte constituents.
The products of the process are a chromium-nickel alloy suitable for reuse in the superalloy industry, electrolytic nickel, electrolytic cobalt, and iron-molybdenum residue. Recovery of the principal elements contained in the scrap is chromium—93 percent, nickel—99 percent, cobalt—96 percent, and molybdenum—92 percent.
From their inception, the industries associated with superalloy manufacture and use have been very conscious of metal recycling and have continually striven to increase product yield and to reuse scrap generated in alloy production and component manufacturing. The principal incentive has been the scarcity and thus the inherently high cost of metals required to give these alloys their unique properties. Occasional shortages in the supply of metals have also stimulated efforts to conserve raw materials. In recent times the supply of some critical metals has been influenced by international political events, in addition to the traditional marketing and technological factors.
In addition, as high-grade domestic ore deposits have been exhausted, the United States has become almost totally dependent on imports for its supply of such critical metals as nickel (Ni), cobalt (Co), chromium (Cr), columbium (Cb), and tantalum (Ta).
These concerns have stimulated renewed interest in metals conservation with the goal of further reducing the quantity of critical metals that are either discarded or downgraded. The present Bureau of Mines directed study is based on the desire of the Federal Emergency Management Agency (formerly Federal Preparedness Agency) to improve recovery of metals, especially chromium, in the superalloy industry. This report describes the results of experiments to test a novel process for recovering metals from superalloy scrap. Evaluation of a second novel recovery process was conducted by Kusik.
Current Chromium Recycling Methods
The quantities of chromium-bearing scrap and waste generated in the United States and disposition of these materials have recently been discussed in detail by Curwick and Kusik. The Curwick study showed that the raw materials charged for superalloy melting may contain 37 to 60 percent scrap, depending on the alloy. The study showed that, of the 291 million pounds of superalloy scrap generated in 1976, 62 percent was directly recycled by the alloy producers, 26 percent was downgraded for use in iron and steel, 6 percent was exported, and 6 percent was lost through service wastage or discarded in landfill. For the most part, it can be assumed that the scrap that is now directly recycled or exported is efficiently used. The material that is now considered waste is usually severely contaminated; consequently, much of it would require specialized and costly, though technologically feasible, methods for recovery. However, the material that is now downgraded provides the basis for significant improvement in recycling efficiency. The reasons for downgrading are numerous: Alloy is too complex for remelting into any other alloy; product quality specifications limit quantity of scrap remelted; scrap from dismantled fabricated components contains unwanted metals; there is contamination from tramp elements in coatings and brazes; scrap contains too much oxide or sulfide for induction melting, etc. Clean solid scrap that is downgraded is usually charged directly into alloy-melting furnaces. Less desirable forms of scrap, including turnings, grindings, and ladle skulls, are frequently melted by a refiner into a secondary alloy pig, which is then charged to an alloy-melting furnace. In either case, the more readily oxidizable elements are deliberately or inadvertently combined with the slag and lost. Valuable elements frequently lost in this manner include tantalum, columbium, titanium (Ti), tungsten (W), molybdenum (Mo), aluminum (Al), and chromium. Cobalt is not oxidized but is often lost through dilution in steels where it is a relatively ineffective alloying element.
Treatment of Superalloy Scrap to Recover Individual Elements
Scrap that is directly recycled by the superalloy producers is usually carefully sorted according to form and grade. Scrap that is downgraded is often mixed and contaminated so that a simple recycling process dealing with any quantity of material would have to accept a feed with a variable composition and would produce a product with composition unlike that of any alloy now produced. The market for such a secondary product is likely to be quite restricted. Consequently, most of the procedures that have been proposed in the past for dealing with these materials have involved at least partial separation of the elements. A variety of processes have been tested or proposed to recover some of the elements in separated form. No process has been identified that economically recovers all of the elements contained in the feed. Most approaches depend on chemical methods for separating the elements, although an initial melting operation has often been used to homogenize the feed, obtain a partial separation of elements, and produce a more readily leachable granular product.
In the least selective processes, such as those currently used by many secondary alloy producers, the charge is melted and the metal bath is severely oxidized until a relatively pure alloy containing Ni, Co, Fe, and sometimes Cr remains. This material is then cast into pigs or shot and sold as a master alloy. As a refinement of this procedure Goto (12) oxidized the bath until all Cr and Fe were removed and then added S to form a matte. The matte was then treated using mineral-processing techniques employed widely in the primary nickel industry to separate nickel and copper sulfides.
A more selective separation was obtained by Aue in which only very reactive elements such as aluminum and titanium were oxidized. The bath was then saturated with carbon and granulated to promote rapid dissolution in the subsequent chlorine leach. This carburizing treatment facilitated separation of Ni, Co, and Fe from Cr, Mo, W, Cb, and Ta, because the latter elements were bound as unleachable carbides. The transition metal carbides can be separated with some difficulty using a caustic fusion and water-leaching procedure. Variation of the caustic fusion process has also been used by Okano and Petrova to extract Mo and W directly from raw particulate superalloy scrap.
Although pyrometallurgical refining provides metallurgical advantages in separation of elements from superalloys, the operations are both capital and fuel intensive. Consequently, some investigators have relied on direct leaching [Brooks, Kruglikov and Van der Meulen] or have used melting only as a preliminary homogenization and granulation step [Baggott and Kawakami]. Following complete leaching in either chloride or sulfate solutions, the above procedures depend on a complex series of selective precipitation, solid or liquid ion exchange, or selective electrolysis steps to produce pure metal products. There are many problems associated with the hydrometallurgical approach, especially in the efficiency of solid liquid separations; however, there currently are no alternatives if complete elemental separation is desired.
One other procedure that provides partial elemental separations is high-temperature chlorination to produce gaseous metal chlorides that can be selectively condensed. This process separates Ni and Co from Fe, Cr, and the refractory metals, but aqueous chemical methods must be used for final separations and purification.
- Fluidized-Bed Roasting of Chromium Sulfide
- Reactor Design
- Effect of Water Content
- Effect of Initial Particle Size
- Aluminothermic Reduction to Chromium Metal
- Desulfurization
- Leaching Nickel Scrap
- Batch Chlorine Leaching Tests
- Effect of Copper Activation
- Effect of Redox Potential Control
- Copper Removal
- CCD Countercurrent Leaching System
- Raffinate Purification
- Continuous Leaching Tests
- Cleaner Leach of Chromium Concentrate
- Direct Leaching of Superalloy Matte
- Cobalt Extraction from Scrap Alloy
- Cobalt Solvent Extraction
- Electrowinning
- Recovery of Metals
Technical Approach for this Process
The approaches described above have emphasized recovery of either Ni and Co or Mo and W, sometimes at the expense of recovery of other metals. Chromium, especially, has been viewed with ambivalence, perhaps because it is so readily available as ferrochromium. However, with growing concern for the long-range stability of chromium supplies and the relatively high prices of the pure chromium needed for superalloy melting, greater attention to recovery of this element while simultaneously achieving high recovery of the other valuable elements is justified. In the hybrid schemes that use selective oxidation of melted scrap followed by leaching, chromium can be either oxidized to the slag or retined in the metal depending on the degree of oxidation. Both approaches have drawbacks. It is difficult to oxidize all of the chromium with oxidizing substantial quantities of other metals, including nickel, and the resulting complex oxide slag is very difficult to treat. On the other hand, if chromium is present in the metal, it dissolves during the leach and causes great difficulty in solid-liquid separation of hydrolysis products. Other metals such as molybdenum and tungsten may also be present, thereby resulting in an extremely complex hydrometallurgical system.
The process described in this report uses an entirely new approach which avoids the problems mentioned above. A simplified flowsheet for the process is shown in figure 1. The process is based on the following principles.
As in other processes, a blended scrap charge is melted and partially oxidized, resulting in Ti, Al, zirconium (Zr), and hafnium (Hf), but little Cr, being segregated to the slag. The bath is then sulfidized to form a partial matte (mixture of molten metallic sulfides). When solidified, the matte essentially contains three components; nickel sulfide (Ni3S2), chromium sulfide (Cr2S3), and a nickel-rich metal phase. The sulfur content of the matte is critical. At the optimum sulfur level, virtually all of the chromium is contained in the chromium sulfide, and the best separation is possible. The subsequent separation of the metal and sulfides can be achieved by conventional magnetic separation and froth flotation or other mineral-processing techniques. The essentially chromium-free, nickel-rich components are then given a hydrochloric acid-chlorine leach (HCl-Cl2) which leaves elemental sulfur as a recoverable residue. Iron and molybdenum are removed by hydrolysis. Solvent extraction and electrowinning are then employed to produce pure electrolytic nickel and cobalt. The chromium sulfide contains some dissolved nickel, which can be removed by leaching. However, since a chromium metal product containing nickel would probably be acceptable for the superalloy industry, further separation was not extensively investigated. The preferred process involves roasting to a low-sulfur oxide product in a fluidized-bed converter, followed by aluminothermic reduction. The latter process produces a chromium-nickel metal ingot.
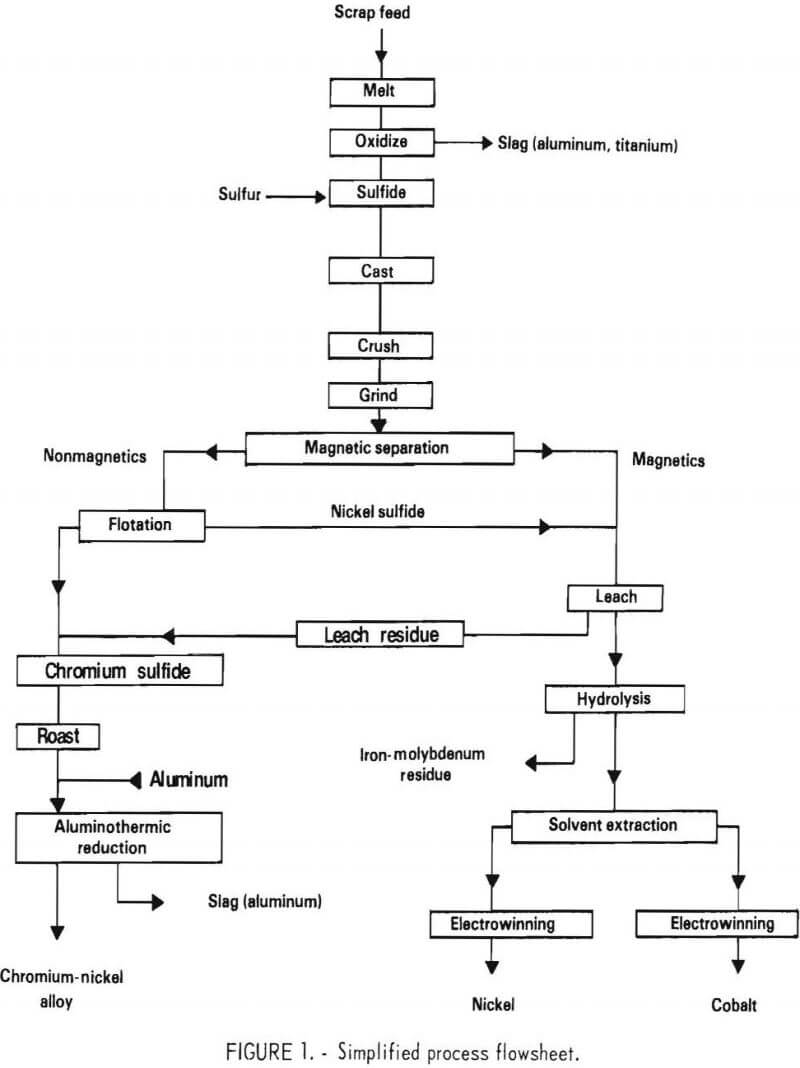
The basic process described above was tested in a series of laboratory experiments. In some cases, modifications of the basic scheme were defined, and limited testing was conducted. Where appropriate, these alternatives are discussed in the text. Based on the results of the experimental work, a proposed pilot plant was designed. The plant was sized to treat 100 pounds of scrap feed per hour. This plant is described in detail in the final contract report.
Experimental Work
The basic flowsheet for the process shown in figure 1 incorporates operations from all phases of extractive metallurgy. The experimental program was subdivided for the sake of expediency into separate tasks with different investigators handling each task. In view of the overall complexity of the process, the tasks will be discussed individually in this presentation.
Superalloy Scrap
Since the purpose of this process was to recover metals from scrap, some introductory discussion of the raw materials selection is warranted.
Composition of Superalloy Scrap
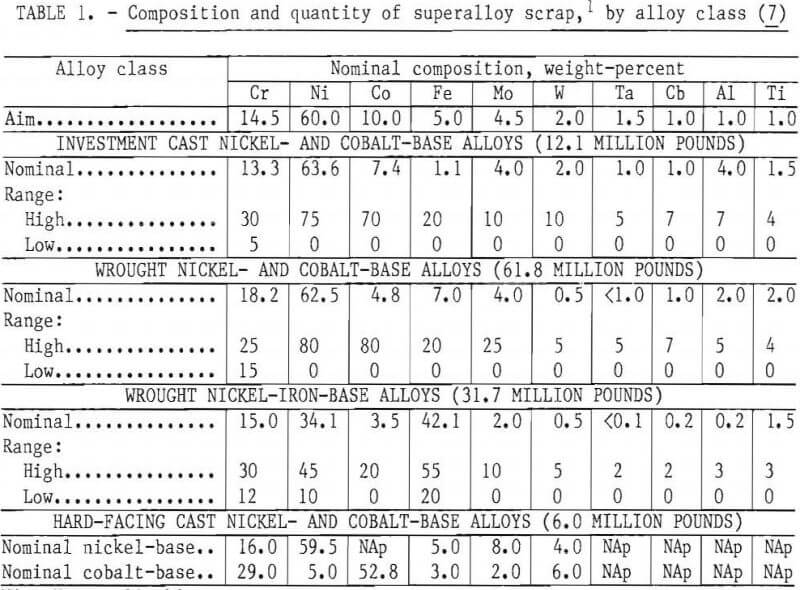
Curwick defines the quantity of scrap generated in the United States by the superalloy producers and users. The separation process under consideration appears to be applicable to four distinct classes of alloys identified in the Curwick study: investment cast nickel- and cobalt-base alloys, nickel- and cobalt-base hard-facing alloys, wrought nickel- and cobalt-base alloys, and wrought nickel-iron-base alloys. The average composition, composition ranges, and quantity of material potentially available for a recovery process are listed in table 1. This information was used to compute a weighted average nominal composition for a hypothetical scrap feed for experimental work.
Form of Superalloy Scrap
A wide range of scrap forms are available as discussed by Cruwick. A detailed discussion of the procedures used by scrap dealers in identifying, sorting, and handling superalloy scrap is given by Cremisio. Dressel provided information on additional forms of scrap. The scrap available for a separation process would range from very high quality “vacuum-grade” material consisting of identified, clean, solid scrap to low-quality “refinery-grade” material such as contaminated mixed alloy grindings. The prices paid for these materials depend on the degree to which they can be directly reused by the superalloy industry and on the recovery of the contained alloying elements. It is probable that a commercial plant would use a scrap feed that is a combination of mixed solid scrap, turnings, grindings, ladle skulls, slag cleanings, and grit from a variety of different alloys.
Raw Materials Used
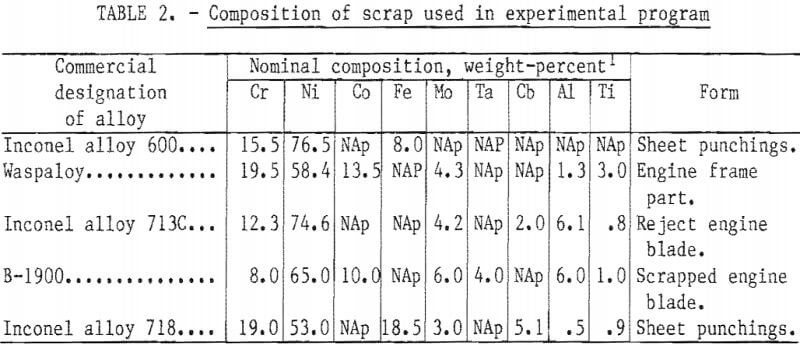
As discussed below, small laboratory induction furnaces were used for alloy melting and refining. To avoid the melting difficulties and composition uncertainties posed by use of the lower grade scrap materials, it was decided to use only clean identified solid material. The first series of small heats in which the base composition was varied over a wide range was made from commercially pure primary metals. In the second phase of the program larger heats were made with the “aim” composition listed in table 1. For these heats, scrap from five selected widely used superalloys was obtained from a commercial scrap dealer. These scrap alloy compositions and forms are listed in table 2.
Melting and Casting
This phase of the program incorporates the basic steps of scrap melting and homogenization, oxidation of the reactive elements, slag removal, sulfidation, casting, and slow cooling. The test work was carried out in three stages:
- Melting and oxidation studies on 12-kilogram heats made from pure elemental raw materials and the “aim” composition,
- sulfidation studies using similar procedures but with a range of base compositions, and
- combined processing using 40-kilogram heats and scrap feed and the “aim” composition. Each of these stages will be discussed in turn.
Oxidation Tests
Oxidation of a molten bath is a convenient tool for refining superalloys provided that the oxidation reactions are selective and controllable and that efficient separation of the oxidation products and metal can be made. Important physical and thermodynamic criteria for selective oxidation include the stability and density of the oxides and the chemical activity of elements in the bath and the slag. There is not sufficient published data to enable prediction of the effect of oxidation on complex superalloys; hence, experimental work was required.
Testing Procedure
A 50-kilowatt coreless induction furnace with a high-density alumina crucible was used for melting heats of approximately 12 kilograms. Commercially pure raw materials were used to prepare alloys having the “aim” composition listed in table 1. The charge, exclusive of reactive metals, was melted under an argon cover and brought to about 2,850° F. The reactive metals (1.0 percent Al, 1.0 percent Ti, 0.25 percent Mn, 0.25 percent Si) were then plunged into the molten charge. After a chemical analysis sample was taken, the bath was oxidized. Three different methods of oxidation were investigated: (1) Blowing a mixture of 75 percent oxygen and 25 percent argon at 30 cubic feet per minute through a lance onto the bath surface, (2) adding granular nickel oxide, and (3) adding ground chromite ore fines. Additions of lime and fluorspar were made to increase slag fluidity. The bath temperature was maintained at about 2,800° F, and samples were taken at various times for analysis. Ultimately the metal and slag were tapped into separate molds and preserved for later use.
Preferred Deoxidant
The first series of test heats showed that granular nickel oxide was the most suitable oxidant; consequently, all of the remaining work was done using that procedure. The oxygen-lancing technique resulted in excessive heating of the bath surface, and the oxygen flow was difficult to control and quantify for the small heats. However, the procedure would be effective for commercial-scale operations. Granular nickel oxide was easy to handle and reacted within a few minutes. Also, this material resembles oxidized nickel alloy waste, which would be a desirable oxidant for a commercial metals recovery plant. Ground chromite ore was tried in one heat to determine whether oxidized chromium, which would be present in a waste alloy oxidant, would be reduced under the test conditions. The experiment showed that chromium was reduced; however, the reaction rate was slow. Chromite ore was otherwise unsuitable as a deoxidant because all of the contained iron and silicon were reduced into the metal phase.
Quantity of Oxygen Required
A number of tests were conducted in which oxide was added gradually and intermediate samples were taken to define the quantity of oxygen required. Oxidation results for Al, Ti, and Ta from several tests are shown in figure 2. Aluminum and titanium were oxidized first. When the concentration of these elements was reduced to about 0.5 percent each, tantalum was oxidized. When the oxygen addition exceeded 1.5 percent, a significant amount of chromium was oxidized. The silicon and manganese contents of the bath did not change during oxidation in these tests. Recovery and distribution of elements between metal and slag are discussed below.
The quantity of oxygen required obviously depends upon the initial concentration of reactive elements in the bath. In these tests, the apparent oxygen efficiency based on the amount theoretically required to oxidize aluminum and titanium was 125 percent. It is presumed that the additional oxygen was supplied by the atmosphere, the original metal charge, and the
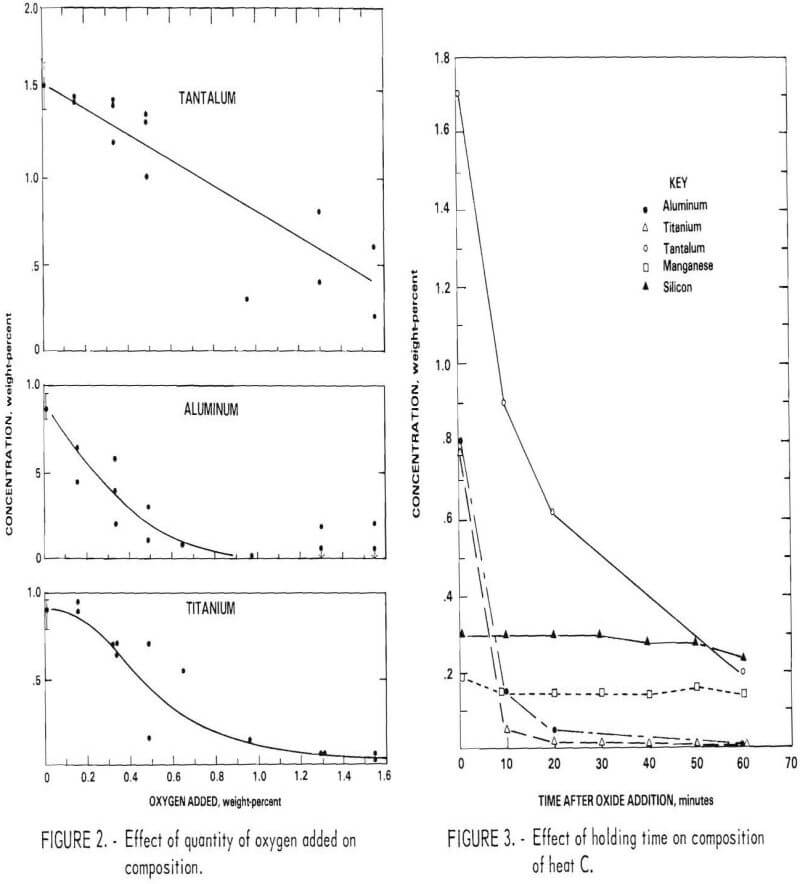
crucible. From the small tests, it was concluded that an addition of oxygen as reducible oxide slightly exceeding the amount predicted from the stoichiometric ratio to convert all Al to Al2O3 and Ti to TiO2 was satisfactory for the larger scale heats.
Rate of Deoxidation
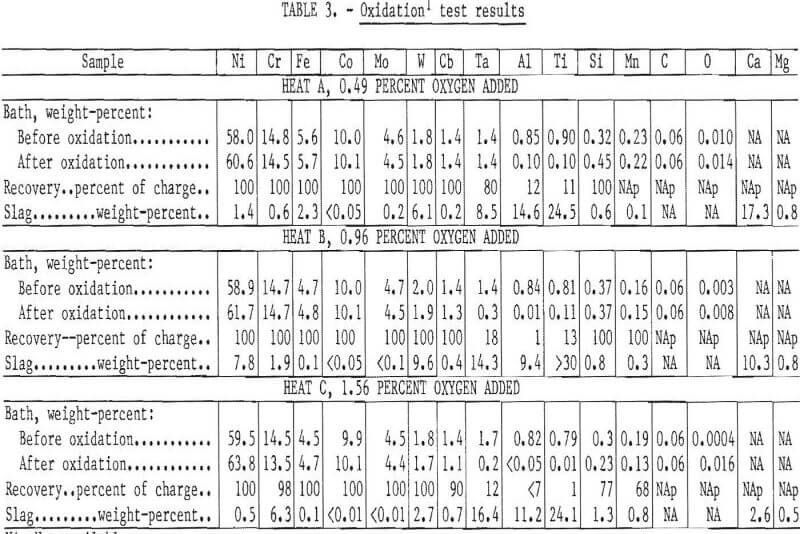
A few tests were conducted in which the bath was held for a period of time following addition of the oxide. Figure 3 shows the concentration of Al, Ti, Ta, Mn, and Si as a function of holding time for heat C (composition shown in table 3). The aluminum and titanium contents of the bath were reduced to a residual level within about 15 minutes. However, the tantalum content decreased gradually over a 60-minute period. Although tantalum is probably oxidized at the same time as the other elements, the solid oxide Ta2O5 has a density nearly that of the liquid and hence is very slowly incorporated into the slag through induction stirring of the bath. The partitioning of tantalum to the slag is likely to be even slower in commercial-scale melts.
Character and Composition of Slag
The oxides of most of the reactive elements are solid at the temperatures used in this work. Consequently it was necessary to add fluidizing agents to form a liquid slag which could be separated with a minimum loss of metal.
Lime (CaO) and fluorspar (CaF2) were found to be suitable fluxes. The amount of flux needed varies with the initial alumininum and titanium content, with an addition of about 10 percent of the expected weight of slag being satisfactory.
Typical slag compositions are listed in table 3. The principal elemental constituents are, as expected, Ti, Al, Ta, and calcium (Ca). One sample had a high nickel content which may have been due to entrapment of metal or unreacted nickel oxide. The high tungsten contents are also believed to be due to entrapment, because very little tungsten was oxidized. The slag taken from heat C, which had the largest oxygen addition, contained 6 percent chromium. The slags produced were rather hard but porous and amenable to grinding for physical recovery of entrapped metal if necessary.
Recovery of Metals
The partitioning of the metals between slag and melt is estimated for three heats in table 3. Considerable difficulty was experienced in establishing a precise material balance because of the uncertainty in actual slag weight and homogeneity. However, it appears that virtually all Al and Ti can be removed with negligible loss of Ni, Cr, Fe, Co, Mo, and W and only a small loss of Cb. Tantalum is distributed in both the metal and the slag with the relative recovery being dependent on quantity of oxygen added and the holding time.
Sulfidation Tests
The addition of sulfur to a molten bath to form either a partial or a full matte is a process widely used in the pyrometallurgical refining of nickel ores. The addition procedures, thermochemistry, and phase relationships of the solidified matte have been studied intensively. However, the matte technology had never before been applied to such complex feeds. Consequently, the existing information and experience on nickel mattes could only serve as a starting point in dealing with superalloy mattes.
Testing Procedure
The initial sulfidation tests were conducted in a 50-kilowatt coreless induction furnace lined with a monolithic high-density graphite crucible. Graphite was used in these tests because it acted as a susceptor to aid in heating and was inert to sulfidation. The charge of approximately 10 kilograms consisted of pure nickel sulfide master alloy (26 percent sulfur) to provide a low-melting heel and either solid or molten oxidized and deslagged simulated superalloy scrap. When the bath was fully molten and homogenized, it was brought to about 2,400° F and sulfur additions were made. To insure high recovery, pure sulfur flour was compacted into small cylinders in a mechanical press. These cylinders were manually held under the bath surface with a graphite rod. Almost 100 percent recovery of sulfur was realized in the small tests using this technique. When the desired sulfur content was achieved, the bath was heated to about 2,750° F, the slag was skimmed, and the matte was poured directly into molds preheated with a gas torch. A variety of refractory molds were used in an effort to minimize the cooling rate of the ingot. Ultimately, a dry silica sand mold backed and covered with high-porosity insulating brick was used. The entire mold was supported by a sand bed and encased in a 1-foot-thick blanket of loose vermiculite. The ingot cooling rate was determined using an embedded thermocouple. All chemical analyses and microstructural studies were performed on samples taken from the solid matte block.
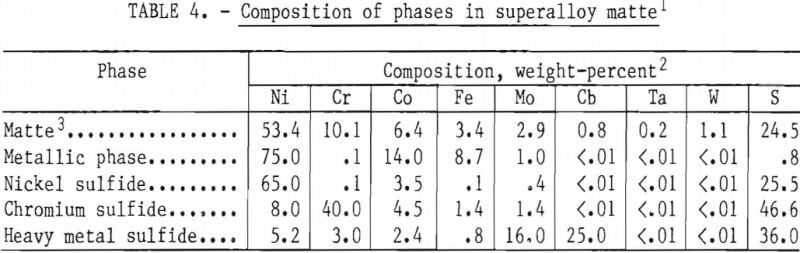
Microstructure of Superalloy Matte
The microstructure of a typical slow-cooled matte sample is shown in figure 4. The composition of the various phases, determined using an electron microprobe analyzer, is shown in table 4. Four distinct phases are visible in this photograph: (A) Light brown nickel sulfide (Ni3S2), (B) dark gray chromium sulfide (Cr2S3), which often has a dendritic structure, which is usually continuous, (C) white or very pale yellow metallic phase, and (D) gray heavy metal sulfide (often MoS2), which is usually in the form of flakes. As discussed below, the composition of each of the four primary phases varies with the base metal feed composition and sulfur content of the matte. The heavy-metal phase is particularly variable and may actually be representative of a series of stoichiometric heavy-metal phases with similar physical characteristics. Heavy-metal carbides and oxides were also observed in selected samples. The grain size distribution is fairly restricted in the sample shown in figure 4; however, within a given matte casting, a wide range of grain sizes as well as occluded grains and highly irregular grain boundaries was observed. The effect of grain shape on phase separation is discussed in a subsequent section.
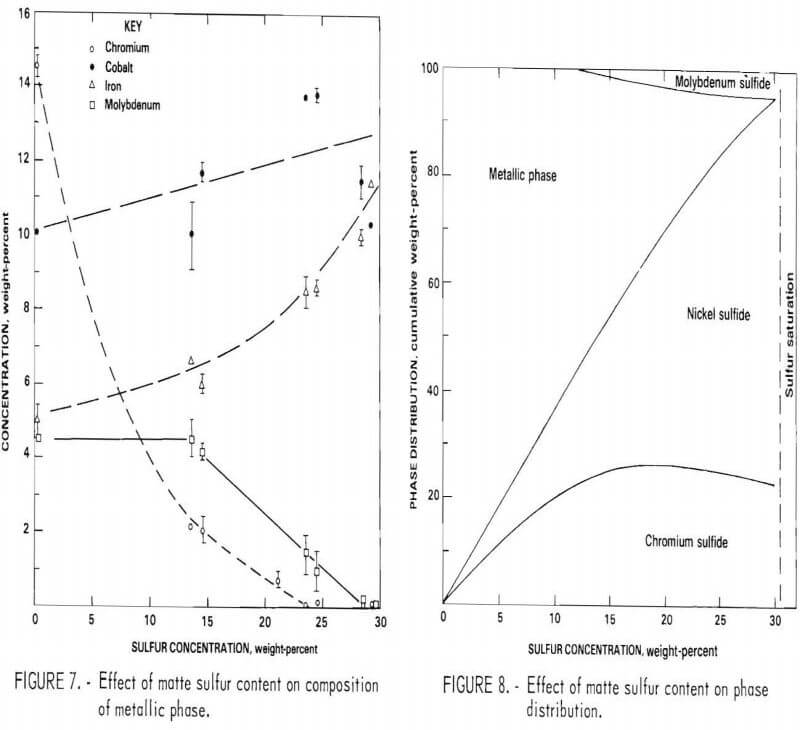
 Effect of Sulfur Content on Element Distribution
Effect of Sulfur Content on Element Distribution
The sulfur content of the matte has by far the greatest effect on the distribution of elements among the various phases. Sulfur affects not only the quantity of the phases but also the concentration of elements, melting point and grindability of the matte, flotation characteristics, volume of leach residue, plant emissions, and many other factors.
A series of heats with similar base composition but having sulfur contents ranging from 13 to 32 percent were prepared. Although the proportion of the phases changed, their morphology was basically the same at all sulfur contents. At the lower end of the range the metallic phase was continuous, and the matte samples were difficult to crush. Very little metallic phase was present in the 30-percent sulfur samples, and nickel sulfide was continuous. These alloys were extremely brittle.
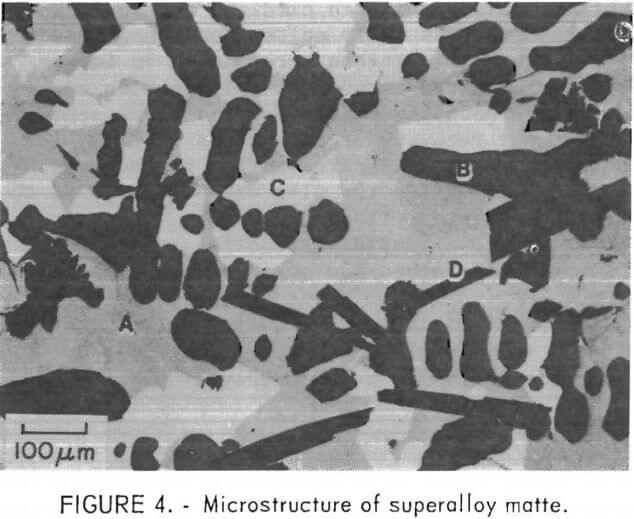
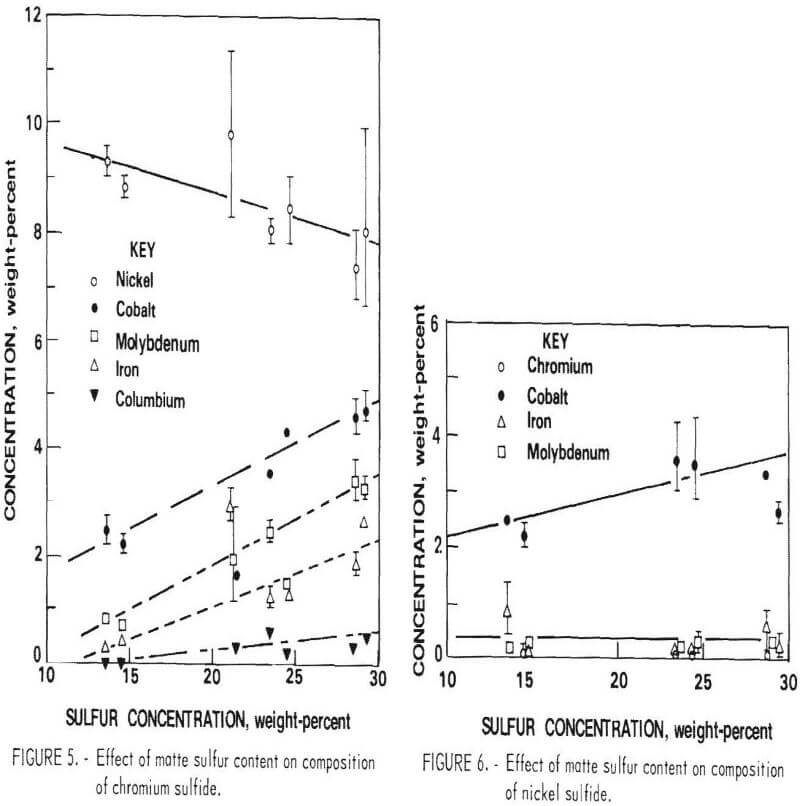
The composition of the three primary phases is shown as a function of sulfur content in figures 5-7. The chromium content of the metallic phase decreases progressively as sulfur is added to the matte until at 20 percent sulfur it is less than 1 percents Nickel and chromium sulfides are both always present as distinct phases in the matte. Note that, although nickel sulfide contains virtually no chromium, a significant quantity of nickel is always present in chromium sulfide. At 15 percent sulfur, about 50 percent of the matte is sulfide. With further increases in sulfur content, the distribution of elements between the phases changes. Heavy metal sulfides are formed, hence the concentration of these elements in the metallic phase progressively declines. At the same time there is a significant increase in the Fe, Co, and Mo content of the chromium sulfide. Figure 8 shows the cumulative weight-percent distribution of the phases estimated from the microprobe results and corroborated by visual observation of microstructure.
The primary goal of the sulfidation procedure is to effectively separate chromium from the other metals. Based on the preceding data, it was estimated that at 15 percent sulfur, the chromium sulfide contains 93 percent of the chromium in the matte. At 20 percent sulfur, 98 percent of the chromium is in this phase. Consequently, the best Cr-Ni separation is obtained at 20 percent or more S. The effect of S on separation of Cr from Co and Mo is more complex, since the concentration of these elements increases with sulfur content. Because of this, the optimum sulfur content appears to be about 17 percent for the feed composition used for these tests. It should be noted, however, that this point was not recognized until late in the program; consequently, most of the matte heats prepared for use in the separation and leaching studies contained about 23 percent sulfur.
Effect of Base Composition
The separation of chromium from the other superalloy constituents for the scrap composite aim composition was very good. However, since heat-to-heat variation in the composition of the scrap feed was likely, an examination of the effect of selected elements on separation was made. As expected, varying chromium and nickel contents did not have much effect on the phase compositions and hence had little effect on the degree of separation.
The element that had the most significant effect was iron. Base alloys containing 10, 15, 20, and 55 percent iron were studied. When more than 10 percent iron was present, additional sulfide phases were formed which produced an extremely complex distribution of elements. In addition to Ni3S2 and Cr2S3, a number of iron-nickel and iron-chromium sulfides of undetermined stoichiometric ratio were observed. This, along with the very high melting point of iron-rich matte, suggested that the average iron content of the composite scrap feed be limited to 10 percent.
The effect of up to 15 percent cobalt and 10 percent molybdenum in the feed was also examined. No new sulfide phases were introduced in either case, and the overall chromium separation efficiency appeared to be unchanged, although the relative distribution of elements within the chromium sulfide phase was somewhat different. Consequently, it appears that iron is the only major constituent of superalloy scrap that should be restricted. It was not possible in this program to determine whether the optimum sulfur level for chromium separation is significantly affected by base composition.
Effect of Cooling Rate on Element Distribution
In the various experiments, matte cooling rate was varied over a wide range. The slowest cooling rate was obtained for a small sample that was program-cooled in a muffle furnace (0.2° F/min). Samples cast in the sand and refractory brick molds described above cooled at rates of 0.4° to 15° F/min depending on their size, degree of mold preheating, matte superheat, etc. Microprobe examination showed no variation in the composition of any of the major phases that could be associated with cooling rate. However, in a test to be described later, a sample of molten matte was granulated in a water stream. This produced a cooling rate of about 20,000° F/min. Microprobe examination of the granulated material showed a small but distinct decrease in the degree of chromium-nickel separation. This finding has implications for an alternative flowsheet using direct leaching of the matte, which is discussed in a subsequent section.
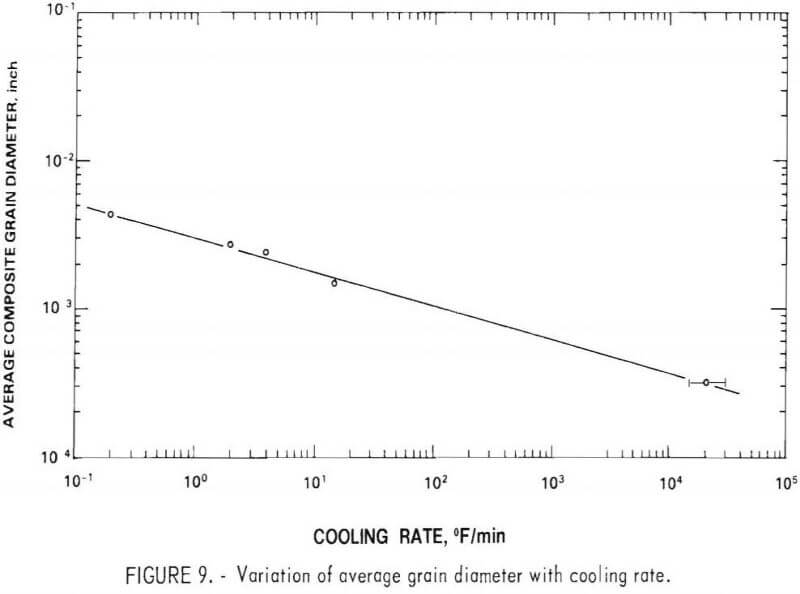
Effect of Cooling Rate on Grain Size
The first matte samples were cast into poorly insulated unheated molds. The resulting grain size appeared to be satisfactory to facilitate phase liberation without excessive grinding. With further testing, liberation was quite difficult and an excessive quantity of fines was generated which adversely affected chromium-nickel separation during froth flotation. Following this observation, a number of improvements in casting procedure were made, which reduced the cooling rate by two orders of magnitude. The average grain size was determined for a number of these heats by a linear intercept method. The relationship between grain size and cooling rate fits a straight line for a log-log plot over five orders of magnitude, including the water-granulated sample (fig. 9). Note that the plot shows average grain diameter for a composite of the four phases (Ni3S2, Cr2S3, MoS2, and the metallic phase). The average diameter for each of the individual phases would be slightly different, but the relationship with cooling rate would be the same.
Effect of Composition on Melting Point of Matte
The melting point of the composite superalloy scrap is between 2,400° and 2,600° F. When sulfur is added, the melting point is depressed significantly. Since nickel is the principal constituent of the matte, the nickel-sulfur phase diagram (1) provides a good first approximation of the melting point characteristics. The eutectic between the metallic and β-nickel sulfide phase (Ni3S2) lies at about 22 percent sulfur and 635° C (1,175° F). The liquidus temperature rises steeply on both sides of the eutectic. Since there are
obvious operating advantages in working with a matte of near eutectic composition, cooling curves of the various heats were examined to determine the critical temperatures. The results indicate that the ternary eutectic for the “aim” superalloy composition lies at about 950° F and 24 percent sulfur. The liquidus temperature for a matte having 17 percent sulfur is about 2,000° F. Although no difficulty was experienced in this program in working well above this temperature during sulfiding and casting, there would be an advantage in the form of energy saving to produce a lower-melting-point matte. The effect of the major elements in the scrap charge on the melting point of the matte, within the range that provides satisfactory chromium separation, is much less significant than total sulfur content. However, very high liquidus and eutectic temperatures (2,200° and 1,270° F) were observed for a 30 percent Fe- 25 percent Ni-17 percent Cr matte.
Integrated Oxidation-Sulfidation Tests
The work discussed in the foregoing sections was performed on 12-kilogram heats using a pure metal charge in most cases. The next step in demonstrating the process was to scale-up and integrate the melting-oxidation-sulfidation¬casting operations. This step was also necessary to produce sufficient matte for test work on other parts of the recovery flowsheet. Actual superalloy scrap was used as the metal feed for the first time in these experiments.
Testing Procedure
Six heats were made in this portion of the program, each having somewhat different procedures to test various aspects of the process. Only a generalized description of the procedure will be provided here.
Melting
In every case a blended charge of 27 kilograms of alloy scrap with the “aim” composition was air-melted with a 300-kilowatt coreless induction furnace in a high-density alumina crucible. The molten charge was brought to 2,950° F and sampled for chemical analysis. Nickel oxide granules were then fed into the bath to oxidize the reactive metals. The oxygen addition of 2.5 percent was selected on the basis of the previous tests and the aluminum and titanium content of the scrap. Lime and fluorspar were added as fluxes along with the nickel oxide. After holding the bath for 20 minutes with furnace power on a low setting, the slag and metal were sampled and the slag was removed.
Sulfidation
Sulfidation was done either in a separate graphite-lined 50-kilowatt furnace (four heats) or in the original melting furnace (two heats). Transfer to the adjacent furnace was done in a preheated refractory-lined ladle. When the two-furnace method was used, 8.5 kilograms of nickel sulfide master alloy was premelted and heated to 1,000° F. The metal charge was tapped into the vessel, and sulfidation was completed by adding pure sulfur briquets in the manner described on page 13. When the sulfidation was done in the original melting furnace, only sulfur briquets were used. The “aim” sulfur content was 20 to 23 percent. In each case, the bath temperature was allowed to fall gradually to the desired tap temperature of 2,250° F.
Casting
Slow-cooled castings were produced from five of the heats. The apparatus and procedure for slow cooling was described earlier. The cooling rate for the 42-kilogram ingots was about 0.4° F/min.
Granulation
Matte samples from three heats were water-granulated. In these tests approximately 10 kilograms of matte was tapped into a small refractory-lined ladle, transported to the granulation station, and poured into a preheated refractory-lined tundish. This tundish was mounted 1 foot above a cylindrical water tank, 6 feet in depth by 3 feet in diameter. The system was designed so that the molten matte stream from a ½-inch-diameter zirconia nozzle intersected a high-pressure water stream. The water flow was about 5 gallons per minute. The solidified matte granules were collected on a screen at the bottom of the tank.
Gas and Dust Monitoring
The melting furnaces were served by hoods mounted on flexible exhaust vents driven by a blower mounted on the foundry roof. The vent was punctured about 20 feet from the furnace and fitted with a bypass pipe. A portion of the effluent was drawn into the bypass using a small fan-driven sampling device. Dust was collected on the filter pads, and gas was collected in a large syringe. The pads were weighed and analyzed by wet chemical analysis. The gas samples were analyzed in a gas chromatograph. The gas velocity in the stack was measured with a velometer.
General Observations
For the most part, the melting behavior of the large-scale integrated heats was similar to that of the small heats. No difficulty was experienced in melting the solid scrap. It was observed that the meltdown proceeded faster when lower-melting-point scrap such as B-1900 and alloy 713C was charged first. Oxidation during air melting was negligible so that little slag was present before the oxide was added.
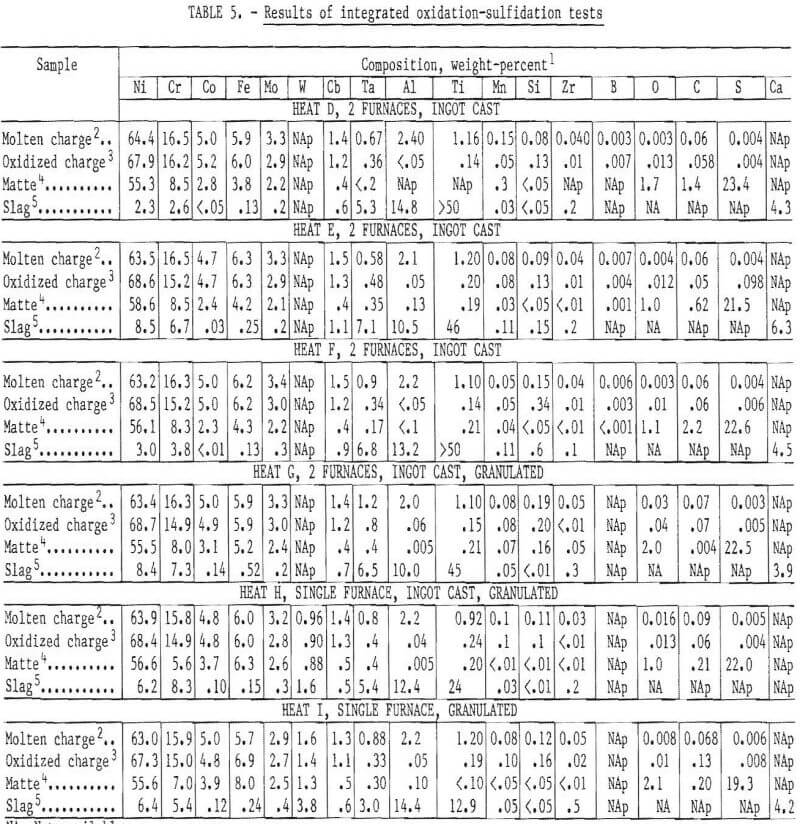
Oxidation using nickel oxide was very quiet and efficient. The light reactive metal oxides separated and floated readily to form the slag, as observed in the small heats. However, tantalum partitioning to the slag was considerably less than before, despite the long reaction period (table 5). Recovery in the oxidized bath averaged 97 percent for Cr, 95 percent for Mo and W, 90 percent for Cb, and 50 percent for Ta. Recoveries of Ni, Co, and Fe were nearly 100 percent.
Scale-up of the sulfidation procedure also presented no new difficulties. Recovery of sulfur was greater than 95 percent. The matte was essentially slag-free, indicating that all metals should have been fully recovered in this operation. However, in a few cases, the matte compositions show variations of major elements before and after sulfidation. This is attributed to difficulty in standardizing chemical analyses and chemical inhomogeneities in the solidified matte. The carbon content of the heats made using the two-furnace practice was 0.6 to 2.2 percent, which resulted in the formation of heavy metal carbides in the matte. The carbon content was 0.2 percent or less when the single furnace practice was used and no carbides were present in the ingot.
Material Balance
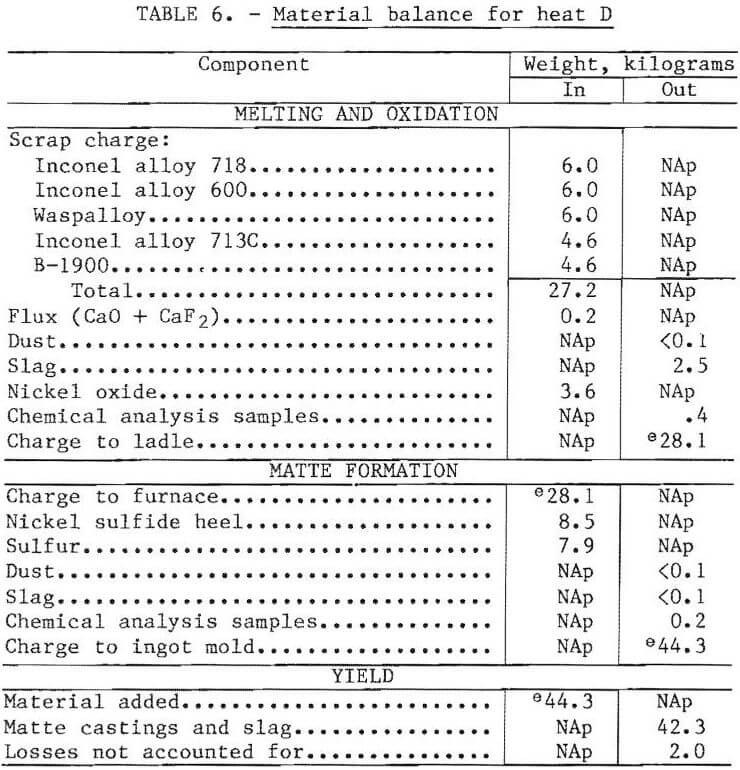
Material balances were prepared for several heats based on the known or inferred weights of the charge and products. The balance for heat D, which was typical of the two-furnace ingot-casting procedure, is shown in table 6. Base metal losses to the slag were about 1 percent of the charge weight. Accounting for other known losses, the amount of material that could not be accounted for was 2 kilograms (4.5 percent of charged weight). This was in the form of spills, splatters, spoon and stirrer rod skulls, ladle skulls, and mold fins. Many of these waste products would be recovered in a commercial operation by recycling back to the melting furnace or incorporation with the crushed matte casting.
Granulation
Although water granulation is often used for fragmenting nickel-copper mattes, its applicability to the superalloy matte was uncertain because of the possibility that chromium might react with water. The granulation proved quite successful, however, and a clean convoluted product was obtained with a particle size range of 0.1 to 0.5 inch. No evidence of hydrogen evolution was observed during granulation, and the final oxygen content of the matte was only slightly higher than that of the slow-cooled matte. The fragmented product was very friable, and the particles could be crushed with the fingers.
The microstructure was similar to that of the slow-cooled matte, although the grains were much finer. The phase composition and grain size of the material were discussed earlier. All in all, water granulation appeared to be a highly desirable means of producing a feed for direct matte leaching. (See discussion in the section on leaching.)
Refractory Wear Tests
As noted, the initial 40-kilogram heats were melted and oxidized in an alumina-lined furnace and transferred to a graphite-lined furnace for sulfidation. This practice was used because these materials were known to be very resistant to attack under the specific conditions. For both capital and operating cost reasons, a two-furnace practice would be undesirable in a commercial plant. Consequently, a series of immersion tests were performed using coupons cut from commercial-grade refractories. The samples were exposed in one test to slag and metal at 2,950° F and in another test to matte at 2,250° F. In both cases the tests were run for 30 minutes with the furnace power at a low setting.
The results of tests are expressed in qualitative terms in table 7. As expected, the oxide refractories showed the greatest resistance in the oxidizing environment, while the carbon-containing refractories were more resistant in the sulfiding environment. Only high-density alumina was resistant to attack in both environments.
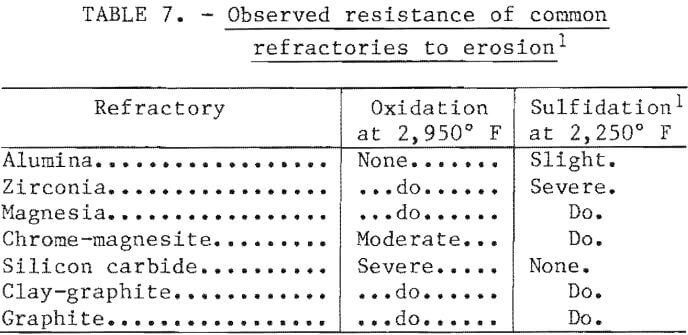
Based on this information, a single-furnace test was conducted using an alumina crucible in a 300-kilowatt induction furnace. Only the surface slag was removed after oxidation, leaving a thin skull of slag on the refractory. The sulfidation was then carried out in the normal fashion with no refractory attack observed. The carbon content of the matte made with this procedure was only 0.2 percent, compared with 2.2 percent using the two-furnace procedure. Consequently, it was concluded that a one-furnace approach would be feasible for a commercial operation.
Furnace Emissions
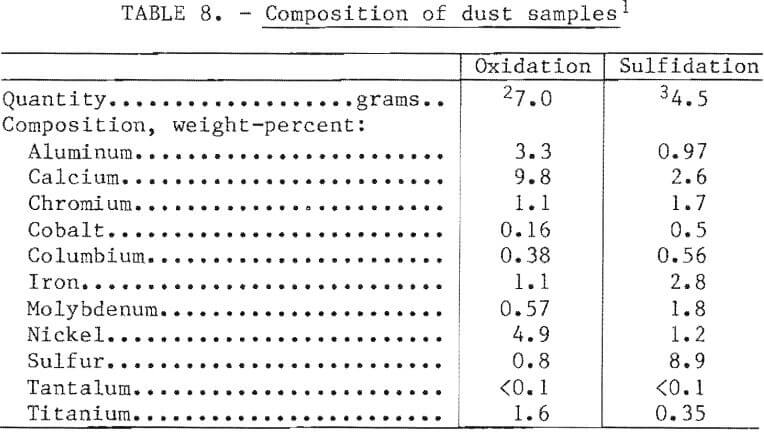
The furnaces used for oxidation and sulfidation were open to the air with exhaust hoods located about 2 feet above the bath to permit access for sampling and charging. There was visible dusting during both operations. The exhaust system appeared to capture virtually all of this dust. Samples of dust taken from the stack bypass were analyzed for the major constituents.
The results are shown in table 8. The dust from the oxidation test appeared to be a mixture of slag, oxidized superalloy, and perhaps some fine nickel oxide. The dust from the sulfidation test was a mixture of oxidized superalloy, slag, and elemental sulfur. The presence of the latter was verified by the bright yellow color of the residue. The volume of dust was extremely small, as would be expected for the charging and melting procedures employed. Pneumatic addition of any particulates, such as superalloy grindings, mill scale, or sulfur, would produce dust loadings of about 0.5 percent of the bath weight, and oxygen lancing could result in losses of as much as 1 percent. In a commercial facility this dust would be recycled back into the melt charge.
Furnace gases were sampled as described previously (under Testing Procedure), Gas drawn from the ventilation system of the oxidizing furnace was indistinguishable from foundry air. The gas from the sulfiding furnace contained some sdlfur dioxide (SO2), but the quantity was less than 1 percent.
Discussion
The experimental work demonstrated the technical feasibility of the process of generating a solid matte with more than 95 percent of the chromium in one phase which, in principle, can be separated from the other phases by physical or chemical means. At 100 percent separation efficiency, a chromium concentrate having 40 percent chromium and a nickel concentrate having less than 1 percent chromium could be produced. The actual separation procedures and efficiencies are discussed in the next section.
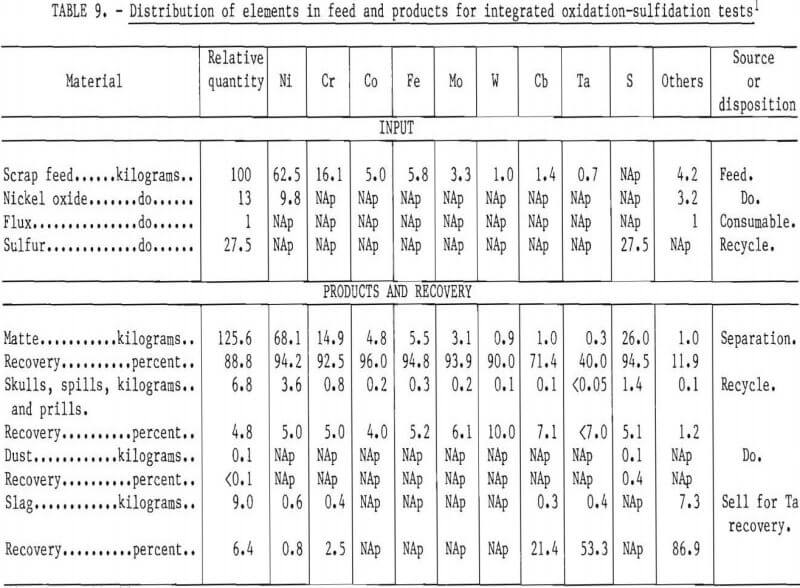
In the laboratory-scale work, recovery of the valuable constituents of the scrap was excellent. Table 9 shows nominal distribution of elements based on a 100 percent scrap charge, the “aim” composition, and nickel oxide and elemental sulfur as the oxidant and sulfidant, respectively. In a commercial operation, skulls and dusts would be recycled so that the only elemental losses would be in the slag. The most valuable constituent of the slag would be tantalum when it was present in the scrap feed. The possibility of selling the slag to a refiner for recovery of tantalum should be explored.
Although it would be desirable to apply the matte separation process to any chromium-bearing feed, it is now apparent that composition restrictions must be applied at least to the composite charge. A composite analysis falling within the following ranges (in weight-percent) should produce a good separation of chromium without severe processing difficulties:
It is possible that the limits for Co and Mo may be higher and therefore Ni lower, but the full range was not covered In this program. It should be obvious that only the composite analysis need fall within the indicated range; any combination of alloys including iron-base superalloys could be used to make up the melting charge.
The scrap feed used in these experiments was all clean, identified, “vacuum-grade” scrap. There is no reason why the process could not be applied successfully to treat other forms of scrap. For example, crushed and de-oiled turnings, skulls, spills, and mixed scrap of all types could be readily melted. Grindings could be treated provided a system for adding them without excessive dusting was used. The tolerance of the process contaminated scrap is unknown. However, certain contaminants such as lead (Pb), bismuth (Bi), tin (Sn), arsenic (As), silver (Ag), and others should be avoided as a matter of principle because of the serious detrimental effect they have on superalloy properties, unless it can be shown that they are effectively removed in the process. Nickel oxide was used as the oxidant in the experimental program because it provided reproducible and quantifiable oxygen additions. In practice, it should be possible to use an oxidized superalloy scrap or waste to supply oxygen. This could take the form of furnace flue dust, mill scale, or even dried pickle sludge. Recovery of elemental oxides reducible by aluminum and titanium should be good.
All experiments were conducted in coreless induction furnaces. This type of furnace is the most convenient for small batch treatments and provides con¬siderable flexibility in control and feeding. The induction furnace would also be suitable for melting in a commercial-scale plant with a single 10,000- pound-capacity furnace being adequate for treating 5,000 tons per year of scrap. Development work for a commercial plant using this melting system would be straightforward. It is possible that other melting furnaces could be used that would reduce energy consumption and possibly increase throughput or reduce the number of plant personnel by operating on a continuous basis. Systems based on submerged arc or plasma heating would be worthy of consideration, but investigation of them was beyond the scope of the present program.
Most of the raw materials were charged by hand in the laboratory work. However, mechanical methods should be used for larger scale systems. Solid scrap would be charged from a tote box or hopper. Particulates such as grindings and oxides could be successfully gravity-fed from an overhead hopper. In this program, sulfur was added as nickel-sulfide master alloy or as briquets. Since sulfur is most readily available as a powder, a method that feeds this material directly would be convenient. Limited experimentation showed that direct addition of powder to the bath surface generated flaring and dusting and resulted in low sulfur recovery. A pneumatic system that injects sulfur powder into the downswirl of a stirred bath should provide adequate recovery, although dusting would be greater than in the laboratory heats.
Although some difficulty was experienced in achieving a sufficiently coarse grain size, this should prove no problem for a commercial-scale plant where ingots of several tons would be cast and allowed to solidify for several days in an insulated holding pit. As an alternative, when direct leaching was employed, the matte could be water-granulated directly in the casting pit.
Separation of Sulfides
In this phase of the operation, superalloy matte was crushed and ground to liberate the phases. Magnetic separation was employed to separate the ferromagnetic nickel-rich metallic phase. The nonmagnetic nickel and chromium sulfides were subsequently separated by froth flotation.
Experimental Procedure
Raw Materials
Samples of broken matte from several heats were used for the initial exploratory testwork. However, the material used for all of the tests reported here was obtained from a single 40-kilogram heat. The composition of this material was 58.7 percent Ni, 8.92 percent Cr, 22.1 percent S, and balance primarily Fe, Co, and Mo. Note that this is a sample analysis; the heat analysis, which is representative of the composition of the entire matte block, differs slightly (table 5, heat D).
Crushing, Grinding, and Magnetic Separation
The matte sample was crushed in stages in a 5- by 6-inch jaw crusher to minus 1 and split into 1 kilogram parcels for storage in a freezer to prevent surface oxidation which could have affected the flotation results. Each sample was crushed to minus 6 mesh just before use.
A 7- by 14-inch laboratory rodmill was used for all of the grinding tests. Samples were wet-ground for various times (10 to 40 minutes per kilogram) using a 50-percent-solids pulp. Magnetic separation was performed manually in a separated cell after completion of the grinding operation. A small laboratory flotation machine was used (without aeration) for these tests. A permanent bar magnet inserted in a plastic casing was employed. Magnetic material was recovered by dipping the encased magnet into the stirred pulp at regular intervals. The magnetic material was released by withdrawing the magnet from the casing. The field strength of the magnet was varied from 0.045 to 0.068 Tesla by inserting spacers between the magnet and the casing.
Chromium Scrap Flotation
Flotation was performed on a small laboratory flotation machine with a 3-liter capacity. The aeration was monitored and controlled with a rotameter. The chemicals and reagents used for the flotation studies are commercially available and are identified by their respective trade or generic names.
The basic flowsheet adopted for the test work is shown in figure 10. This flowsheet is based on the matte separation practice used commercially by Inco at the Copper Cliff Ontario Smelter for treating nickel-copper matte.
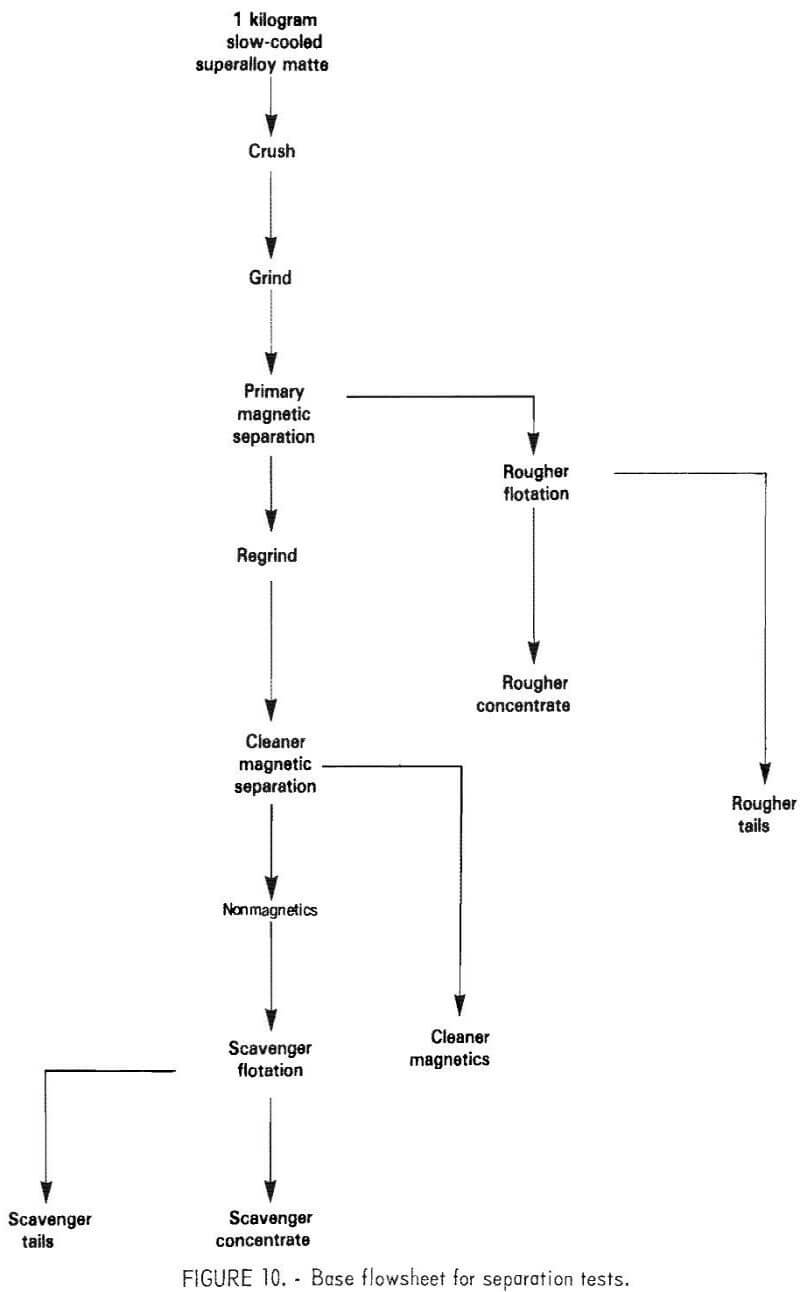
The flowsheet employs a two-stage sequence of grinding, magnetic separation, and flotation. The test conditions were varied in accordance with the overall goals of minimizing chromium sulfide content of the metallic concentrate and maximizing nickel-chromium separation during flotation. Details of variables studies for these tests are described in the following sections. Complete separation of the metallic and nickel sulfide phases was not considered important because they are recombined for leaching.
Determination of Optimum Grinding Time
The grinding time for phase liberation was studied from two aspects: (1) Effect on nickel and chromium recovery in the metallic fraction in the primary and cleaner magnetic separation, and (2) effect on nickel and chromium separation in rougher and cleaner flotation.
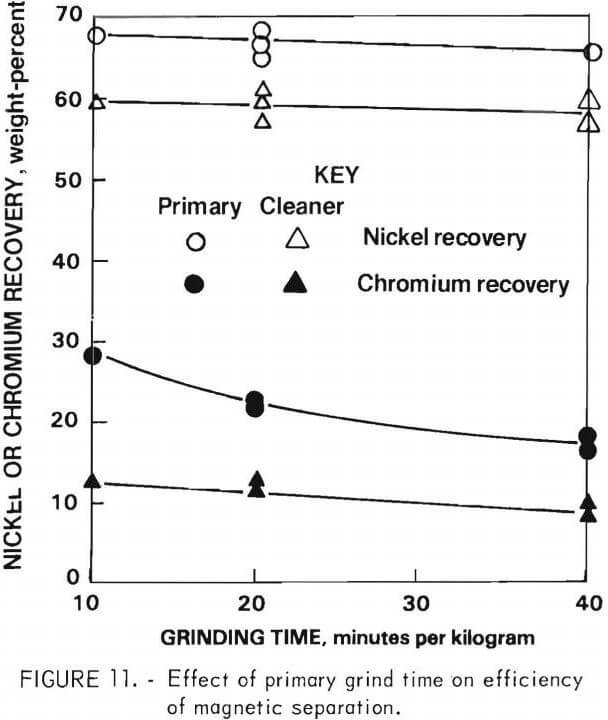
Effect on Magnetic Separation
The effect of time during the primary grind on nickel and chromium recovery in the metallic fraction is shown in figure 11. The objective was to have the lowest chromium content in the fraction “cleaner magnetics,” which is the feed for the leaching circuit. The results show that increased primary grinding reduces chromium in the intermediate “primary magnetics” product. However, increased primary grinding time has a much smaller effect on the reground “cleaner magnetics” product. As might be expected, the total weight of “cleaner magnetics” was not greatly affected by primary grinding time.
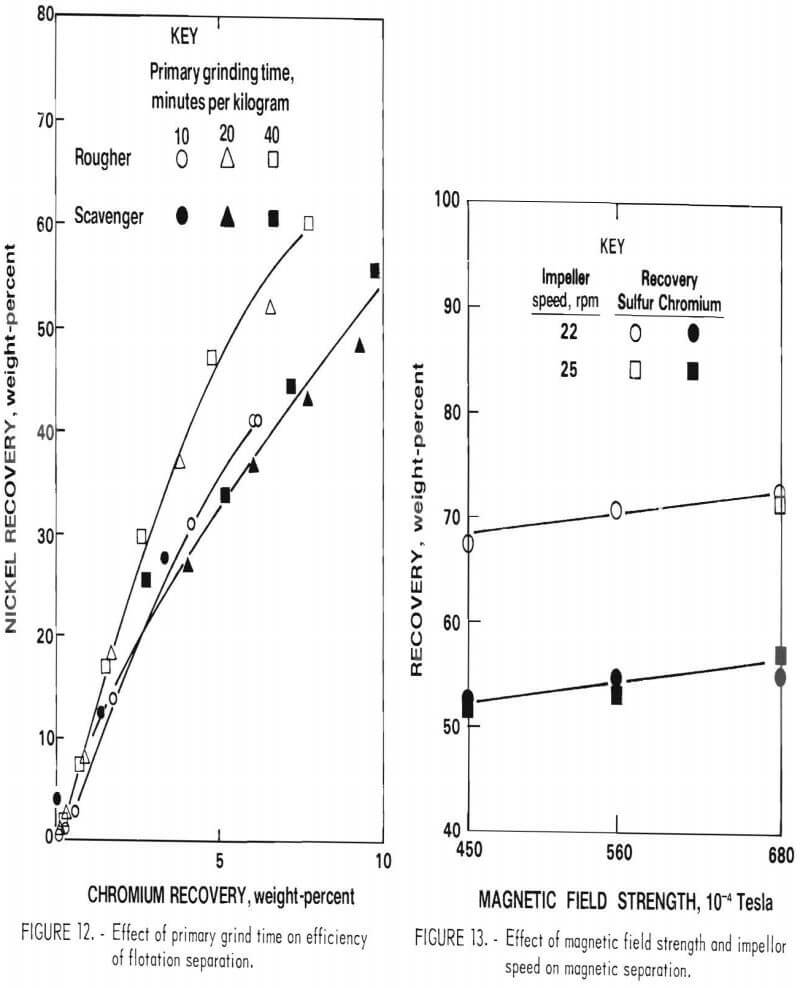
Effect on Flotation Separation
Tests showed that the natural hydrogen ion concentration (pH) of the pulp was 8.5. The initial flotation tests were conducted at this natural pH, using potassium amyl xanthate (KAX) as collector and methyl isobutyl carbonyl (MIBC) as frother. The indicator for effect of grinding time in this case is the separation of nickel and chromium, nickel being recovered in the froth and chromium in the tails. The results presented in figure 12 show a small increase in nickel-chromium separation in the rougher flotation circuit but almost no effect on separation in the scavenger circuit. Analysis of the results of the test indicated that nickel sulfide-chromium sulfide liberation was improved with longer grinding but at the expense of the formation of a higher proportion of hard-to-float nickel sulfide slimes (particles smaller than 10 micrometers).
Based on the above results, grinding times of 20 and 8 minutes per kilogram were selected for the primary and secondary grinding steps.
Chromium Magnetic Separation Conditions
An additional series of tests was run on the cleaner magnetic separation circuit to improve separation of metallics from the sulfides. These tests were run using various magnetic field strengths and impeller speeds for agitation of the ground matte pulp. The results in figure 13 show chromium and sulfur recoveries as a function of impeller speed and magnetic field strength, based on the composition of the cleaner magnetic separation feed. The effect of speed of agitation was negligible within the range tested (22 to 25 revolutions per minute). The effect of magnetic field strength was the inverse of what was expected; for example, there was more nonmagnetic phase in the magnetic fraction at higher field strength. Microscopic examination indicated that this effect was due to the presence of fine particles of metallic phase (<5 micrometers diameter) disseminated within the sulfide grains. Further grinding to free these particles was judged to be impractical.
Flotation Separation of Nickel and Chromium Sulfides
As noted previously, maximum separation of nickel and chromium was sought in the flotation operation. The degree of separation can be expressed in terms of separation efficiency defined as the difference in nickel and chromium recoveries. Complete separation of the elements by flotation giving an efficiency of 1.0 is impossible in this situation because the chromium sulfide phase contains some dissolved nickel. An efficiency of about 0.9 is the theoretical maximum.
It was established early in the test program that the nickel sulfide flotation rate was quite slow at the natural pH of 8.5 using KAX as collector. Also, the nickel recovery was low so that a chromium concentrate containing a high proportion of free nickel sulfide was produced. The following program was designed to improve separation efficiency.
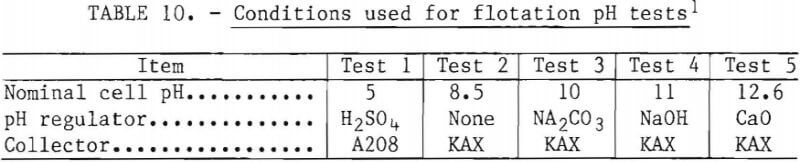
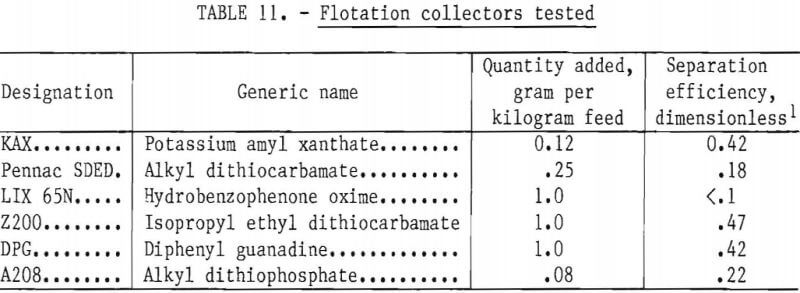
Effect of pH
The flotation conditions used in this study are listed in table 10. The test results are shown in figure 14. The flotation rate of nickel sulfide exceeded that of chromium sulfide over the entire range of pH studied. The best separations were achieved at the natural pH of 8.5 and at pH 10. Based on this information, all subsequent flotation tests were done at natural pH.
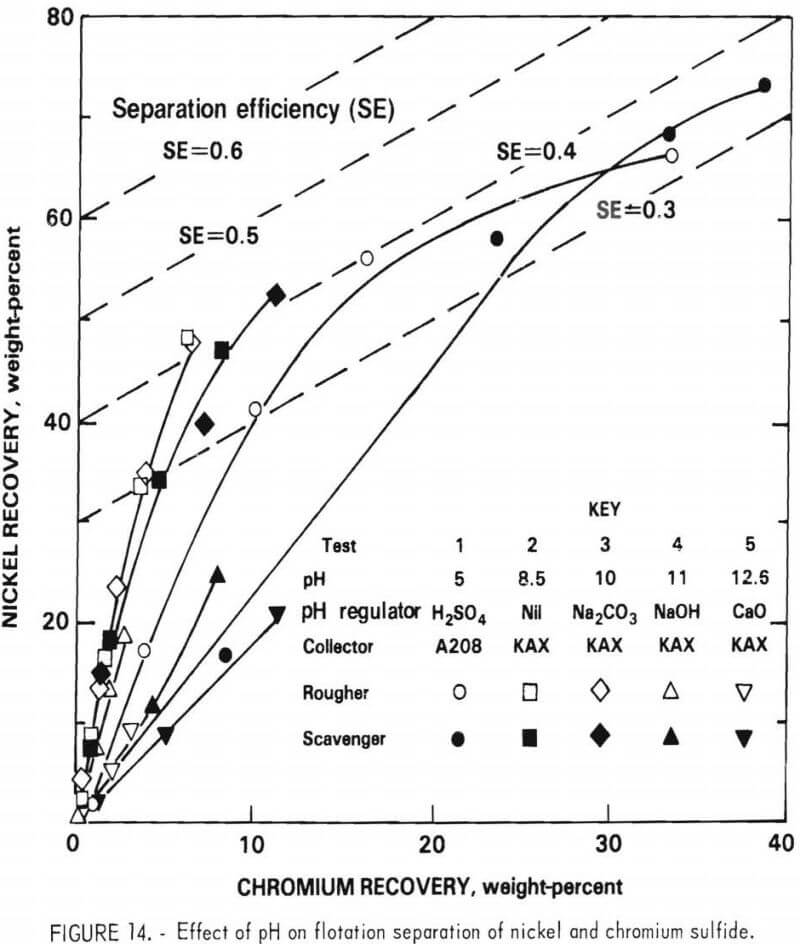
Effect of Collector
The six collectors listed in table 11 were evaluated at natural pH using the rougher flotation feed. The results of these tests are shown in figure 15. Z200 provided the highest separation efficiency, 0.47 versus 0.42 for KAX and DPG. However, owing to its low aqueous solubility, a relatively large quantity of Z200 was required. Larger additions of KAX were also examined, but significantly higher separation efficiencies were not obtained. Both Z200 and DPG were very selective in producing a low-chromium, high-nickel concentrate. However, the overall nickel recovery and flotation rate obtained with these collectory was not as good as that provided by KAX. KAX was ultimately specified as the collector, since it provided the best overall flotation behavior.
 Preflotation Activation
Preflotation Activation
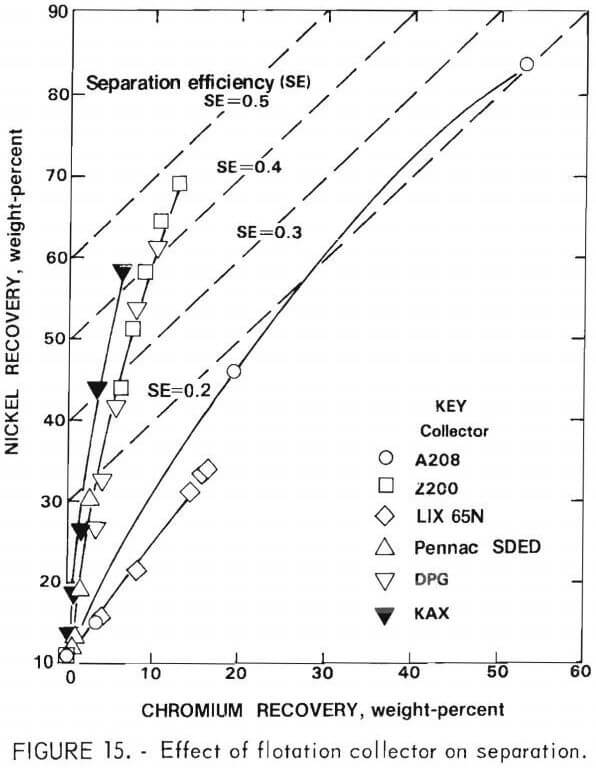
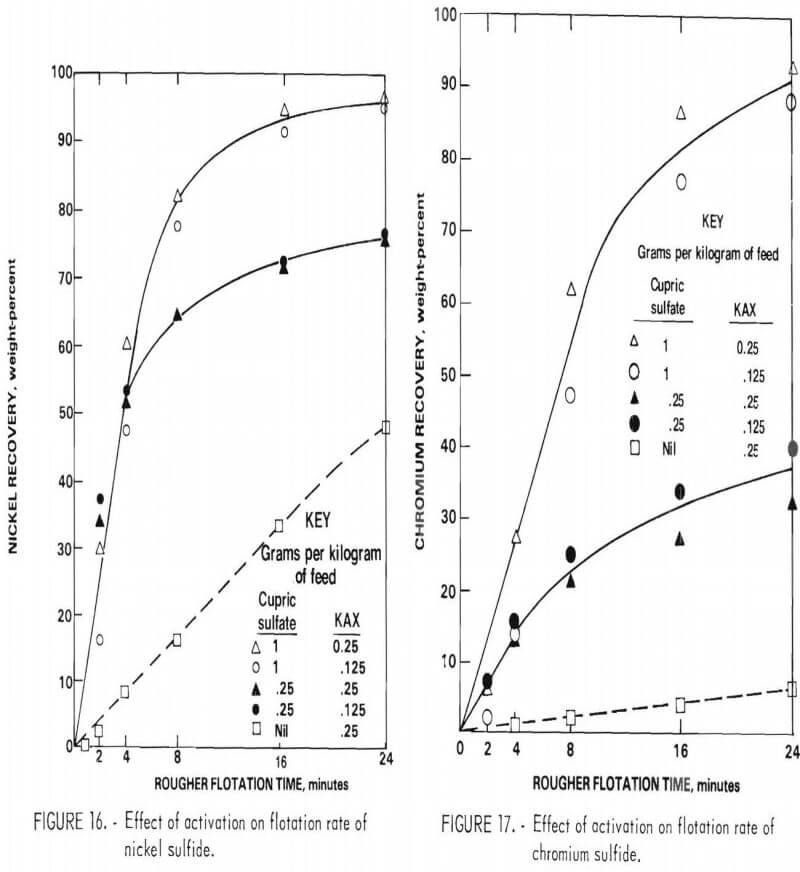
Activation with cupric sulfate (0.25 and 1.0 gram per kilogram of feed) using KAX as collector was studied for the rougher flotation feed. This procedure greatly accelerated the flotation rate of both nickel sulfide and chromium sulfide (figs. 16-17). Unfortunately, there was no significant improvement in separation efficiency. The relatively high nickel content of the chromium sulfide may account for the lack of selectivity of cupric sulfate activation.
Best Conditions
An open circuit material balance is shown in figure 18 for the best separation obtained in this work. The specific test conditions are enumerated in the figure. Combination of the rougher and scavenger flotation tails gives a chromium sulfide concentrate of 34 percent of the original matte weight; 81.4 and 21.0 percent, respectively, of the contained chromium and nickel are recovered. This concentrate is composed of about 50 volume-percent each of chromium sulfide and nickel sulfide.
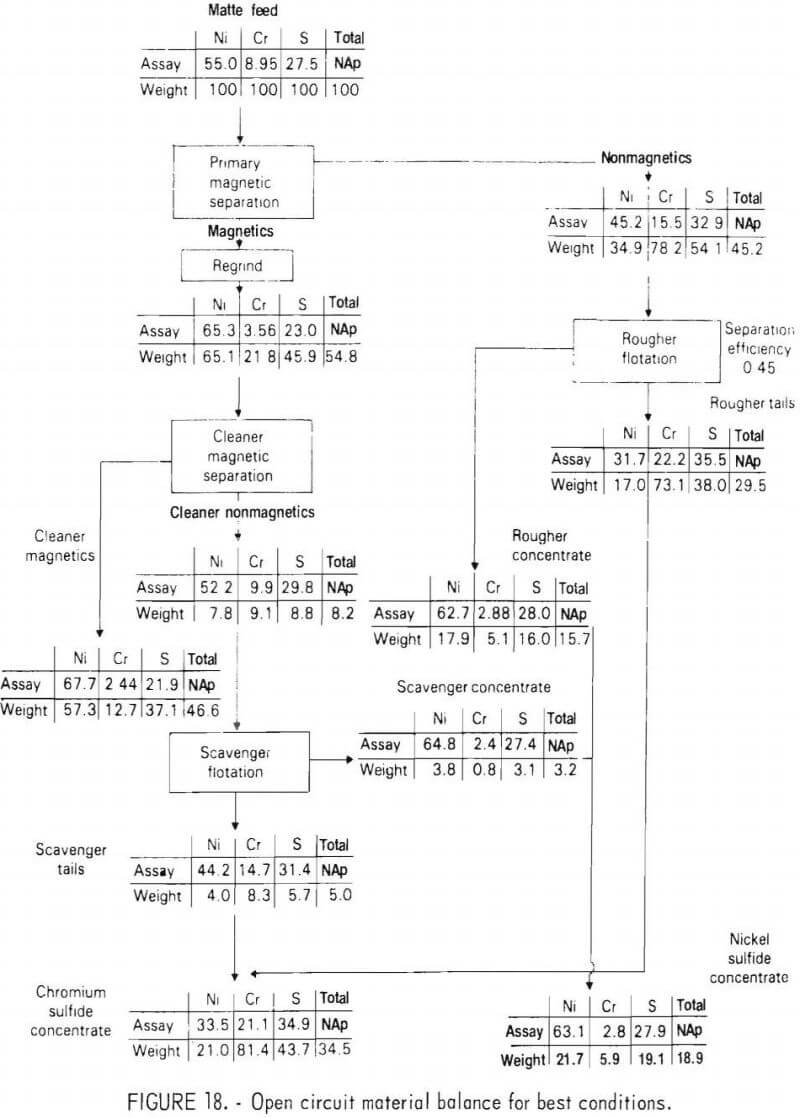
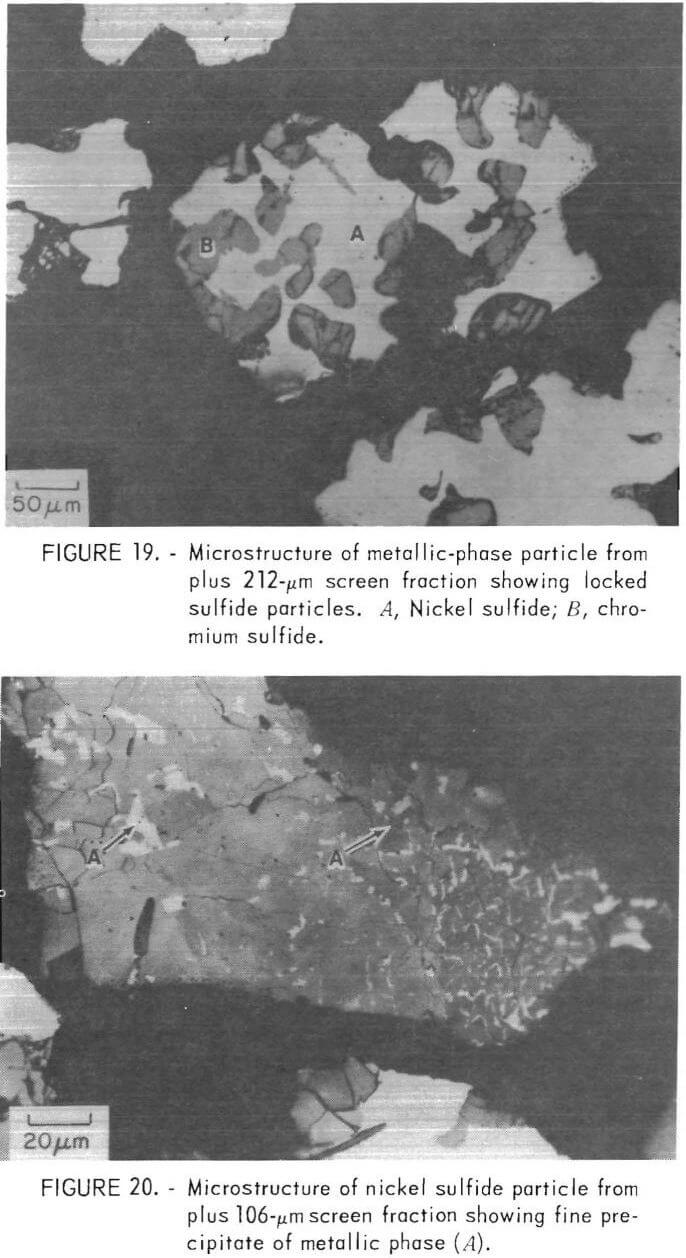 Mineralogical Studies
Mineralogical Studies
During the program, several mineralogical studies were made of the ground matte using optical microscopy and electron beam microprobe analysis. These studies showed that phase liberation was not as good as had been expected on the basis of the original matte grain size and shape. Figure 19 shows a representative metallic phase grain from the plus 212 micrometer screen fraction. The grain contains numerous locked sulfide particles. These particles could not be liberated through additional grinding without creating an excess of sulfide phase fines. It was also noticed that extended grinding resulted in deformation of the ductile metallic phase to the extent that some sulfides were actually enveloped by the metallic phase.
Figure 20 shows a small liberated nickel sulfide particle which contains a significant amount of metallic phase. This precipitate probably results from solid state rejection of nickel from solution during cooling, as would be expected from the nickel-sulfur phase diagram (fig. 10). The magnetic nickel phase would be weakly attracted by a magnetic field, thus reducing the efficiency of the magnetic separation step.
Discussion
It is evident from the preceding sections that the mineral separation section of the flowsheet is not as efficient as anticipated. Two major contributing factors were (1) relatively poor liberation of primary sulfide particles from the metallic phase during grinding and (2) solid state precipitation of metallic phase particles within the nickel sulfide grains. Both of these problems would be alleviated but not necessarily eliminated by the slower cooling rate that would be achieved with large ingots in a commercial-scale plant.
Problems were also encountered in the flotation separation that are unrelated to prior processing history. Because of their extreme brittleness, the sulfides are prone to formation of fines during grinding. In the case of nickel sulfide, the fines are difficult to float without the use of activators. Unfortunately, chromium sulfide, perhaps because of its dissolved nickel, was also activated by copper sulfate. Consequently nickel-chromium separation was not as good as desired. It should be pointed out, however, that there is no body of experience to draw on for flotation of chromium sulfide. It is possible that further experimental work may define reagents and processing systems that will permit a closer approach to the theoretical maximum nickel-chromium separation efficiency of 0.9.
It was noted in an earlier section that phases containing the heavy metals may also be present in the matte. The phase occurring in the greatest quantity was a molybdenum-tungsten sulfide. The amount of this phase increases with the sulfur content of the matte. In the magnetic separation circuit discussed above, M02S reports to the nonmagnetic sulfide fraction; limited testing using KAX as collector showed that Mo2S floats more rapidly than Ni3S2 and consequently is almost entirely contained in the nickel concentrate. This distribution is desirable from the standpoint of maximum recovery of molybdenum in the leaching circuit as discussed below.
The best chromium concentrate produced in the laboratory work contained approximately equal amounts of nickel and chromium sulfides. If further test work does not significantly improve the flotation separation efficiency, alternative means for further concentrating the chromium sulfide must be used. One method is to use a partial leach to selectively dissolve nickel sulfide. This procedure is discussed in the leaching section of this report.
https://www.911metallurgist.com/how-to-recover-chrome-metal-from-scrap-alloy/
