Table of Contents
METHOD for SEQUENTIAL AND RESIDUAL COPPER ANALYSIS
PURPOSE: To determine, through sequential dissolution, the probable copper distribution by copper mineral in a sample.
| SCOPE: | Matrix: | Copper ores |
| Analyte: | Copper (Cu) | |
| Range: | <1-20%. |
Accuracy/Precision: This method is not intended to be accurate since there is not one standard procedure for the determination of non-sulphide copper. Results determined by the non-sulphide soluble copper digestion procedure, indicate to the percentage of oxide copper content minerals as well as chalcophyrite. The cyanide soluble copper analysis results determined by this procedure, indicate to the amounts of non-chalcopyrite sulphide mineral content such as Chalcocite and Covellite in the sample. Residual copper analysis is presumed to indicate the content of chalcopyrite in the sample.
At no time can the combined values of the three determinations exceed the determined total element content. This procedure is precise in that duplicate analyses are within acceptable ranges of agreement.
Interferences: There are no known interferences in this method.
| SPECIAL EQUIPMENT: | Analytical Balance |
| Filter Racks/Funnels | |
| #1 Whatman Filter Paper — 12.5 cm | |
| AAS | |
| Standard Laboratory Equipment |
Chemicals and Reagents
- All Chemicals are of Reagent Grade unless indicated otherwise.
- Sulphuric Acid (H2SO4)
- Sodium Cyanide (NaCN)
- Hydrochloric Acid (HCl)
- Nitric Acid (HNO3)
- Bromine (Br2)
Solution Preparation:
- Sulphuric Acid (H2SO4) 5%
- Sodium Cyanide (NaCN) 10%
- Metal Standard Working Solutions — matrix compatibility
- Step 2.0: 0.5% H2SO4
- Step 3.0: 1.0% NaCN
- Step 4.0: 10.0% HCl
| REFERENCES: | Method Statements, General References | |
| General Work Order | ||
| Assay Request / Report Form | ||
| Assay Worksheet | ||
| Calibration Log for AAS | ||
| Reference Pulp Standard Values | ||
| Method Statements – various | ||
| The Sequential Copper Analysis Method — Geological,Mineralogical and Metallurgical Implications |
||
PROCEDURES:
1.0 Preparation Weighing:
1.1 Weigh homogenized samples as required.
1.2 Weigh 0.2000 — 1.0000 g (+0.0010 g) of sample into a 200ml beaker, unless other information is provided.
1.3 Ensure that necessary solutions are prepared in suitable volume.
1.4 Prepare a reagent blank for each of the three digestion processes.
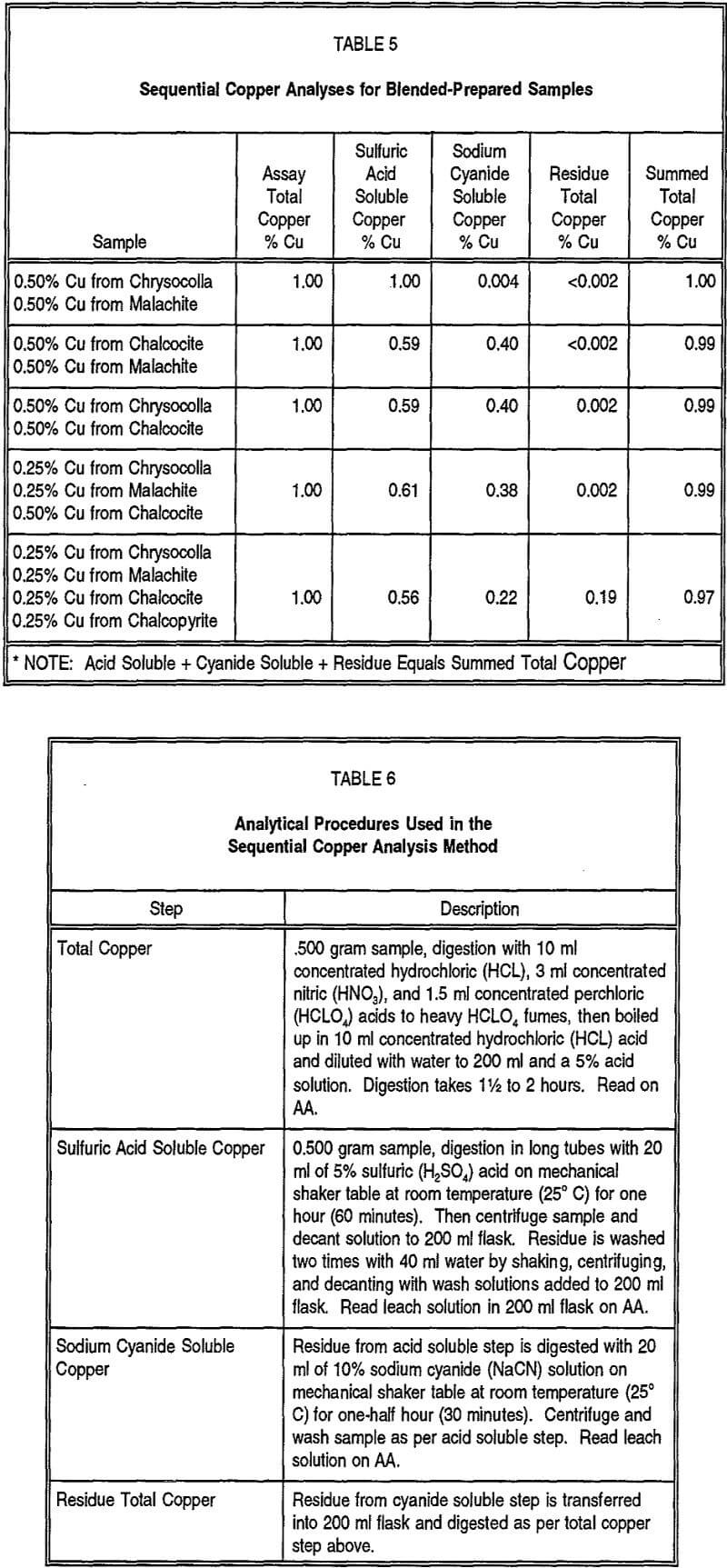
2.0 Non Sulphide Soluble Copper:
2.1 Add 20 ml 5 % H2SO4, wetting the entire sample.
2.2 Leach at room temperature for 60 minutes, agitating every 15 minutes.
2.3 Filter through a 12.5 cm, #1 Whatman paper into a 200 ml flask.
2.4 Rinse the residue well three times with de-ionized water. Save the residue if cyanide soluble bulk copper is required.
2.5 Bulk the flask to 200 ml, shake well and read on the atomic absorption spectrometer using the appropriate copper metal standards in a 0.5% H2SO4 solution.
2.6 If the absorbance of a sample is above the highest concentration standard, then dilute the sample 10x with 0.5% H2SO4 solution.
3.0 Cyanide Soluble Copper:
3.1 Wash the filter residue from the acid soluble procedure into a clean 200 ml beaker with water.
3.2 Allow settling at least one hour before decanting off all possible water.
3.3 Add 20 ml 10% NaCN to the residue in the beaker.
3.4 Leach at room temperature for 30 minutes, agitating every 15 minutes.
3.5 Filter through a 12.5 cm, #1 Whatman paper into a 200 ml flask.
3.6 Rinse the residue well three times with de-ionized water. Save the residue if residual copper is required.
3.7 Bulk the flask to 200 ml, shake well and read on the atomic absorption spectrometer using the appropriate copper metal standards prepared in a 1.0% NaCN solution.
3.8 If the absorbance of a sample is above the highest concentration standard, then dilute the sample 10x with 1.0% NaCN solution.
4.0 Residual Copper:
4.1 If Total Copper has been determined and Residual Copper analysis is not required, proceed to Step 5.0 to calculate the residual copper concentration; otherwise, proceed to step 4.2.
4.2 Wash the filter residue from the cyanide soluble procedure into a 200 ml flask with minimal water (30 – 40 ml).
4.3 Add 15 ml HCl to all samples.
4.4 Place flasks on hot plate and heat to boiling for 15 minutes.
4.5 Remove from heat. Add 5 ml water and 10 ml HNO3.
4.6 Allow samples to cool 5 minutes. Add 0.5 ml bromine.
4.7 Allow bromine to react with the samples for 5 minutes. Return flasks to hot plate. Digest for 20 minutes or until clean fumes.
4.8 Remove the flask from the heat. Add 15 ml HCl. Add water to all flasks to just below the volume mark.
4.9 Cool in water bath. Bulk to 200 ml, then stopper and shake well.
4.10 Read on the atomic absorption spectrometer using the appropriate copper metal standards.
5.0 Data and Calculations:
5.1 Refer to procedures appropriate to AAS for calibration and data analysis.
5.2 Set-Up of the AAS
5.2.1 Calibrate using the Metal Working Standard Solutions.
5.2.2 Read directly against the Metal Working Standard Solution concentrations.
5.3 Residual copper calculation:
Residual copper = Aqua Regia Copper Concentration — (Sulphuric Acid Copper Leachable Concentration + Sodium Cyanide Leachable Copper Concentration)
6.0 Solution Preparation:
6.1 Leach Solutions:
6.1.1 Prepare the necessary leach solutions.
6.2 Metal Standard Working Solutions.
6.2.1 Prepare Metal Working Standard Solutions to match the matrix solution of the digestion processes.
Sequential Copper Analysis Method
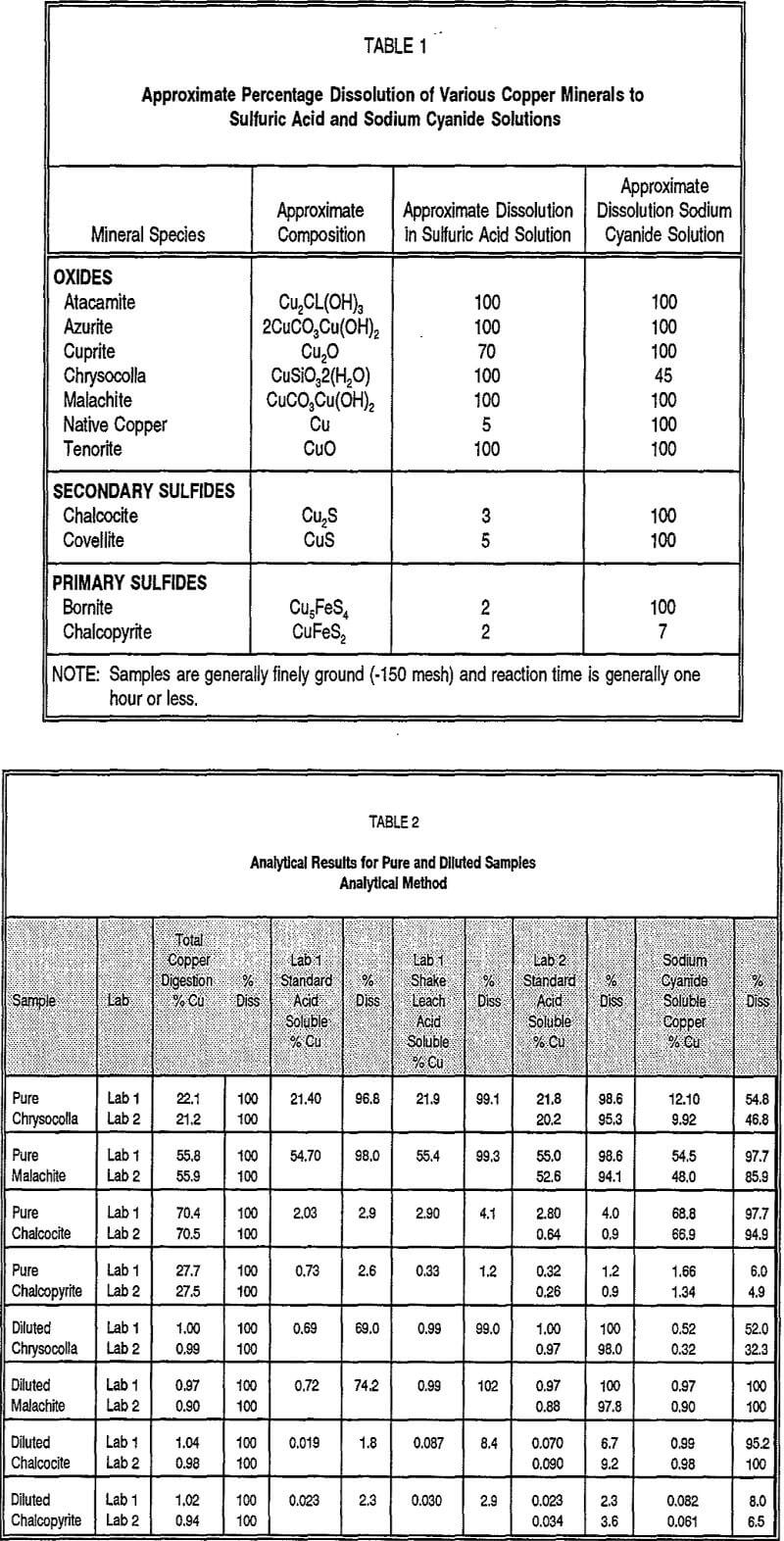
Investigations conducted on various ores containing the copper minerals included in Table 1, in both metallurgical test columns and under pad and heap-leaching conditions, have demonstrated their dissolution behavior and provide the metallurgical basis for development and application of the sequential copper analysis method. Under these conditions, it has been found that sulfuric acid leaching is generally quite successful in recovering a large percentage of copper from copper oxide minerals, closely approaching the values for the specific minerals as shown in Tables 1 or 2 over relatively short leaching times less than 100 days. In contrast, leaching with sulfuric acid under both laboratory and commercial-scale conditions dissolves only very small amounts of secondary and primary sulfide minerals. Similar lab and commercial-scale testing utilizing oxidizing agents, such as ferric iron, in combination with sulfuric acid have demonstrated that both oxide and secondary sulfide minerals are quite thoroughly leached over longer periods-exceeding 100 days. With the exception of chrysocola, leaching behavior of copper-bearing minerals under ferric-iron/sulfuric acid leaching is generally closely modelled by dissolution with sodium cyanide of assay-type samples. Primary sulfide minerals, such as chalcopyrite, are essentially unleachable under laboratory or commercial-scale conditions using any of the previously mentioned conditions or reagents, even over very long periods.
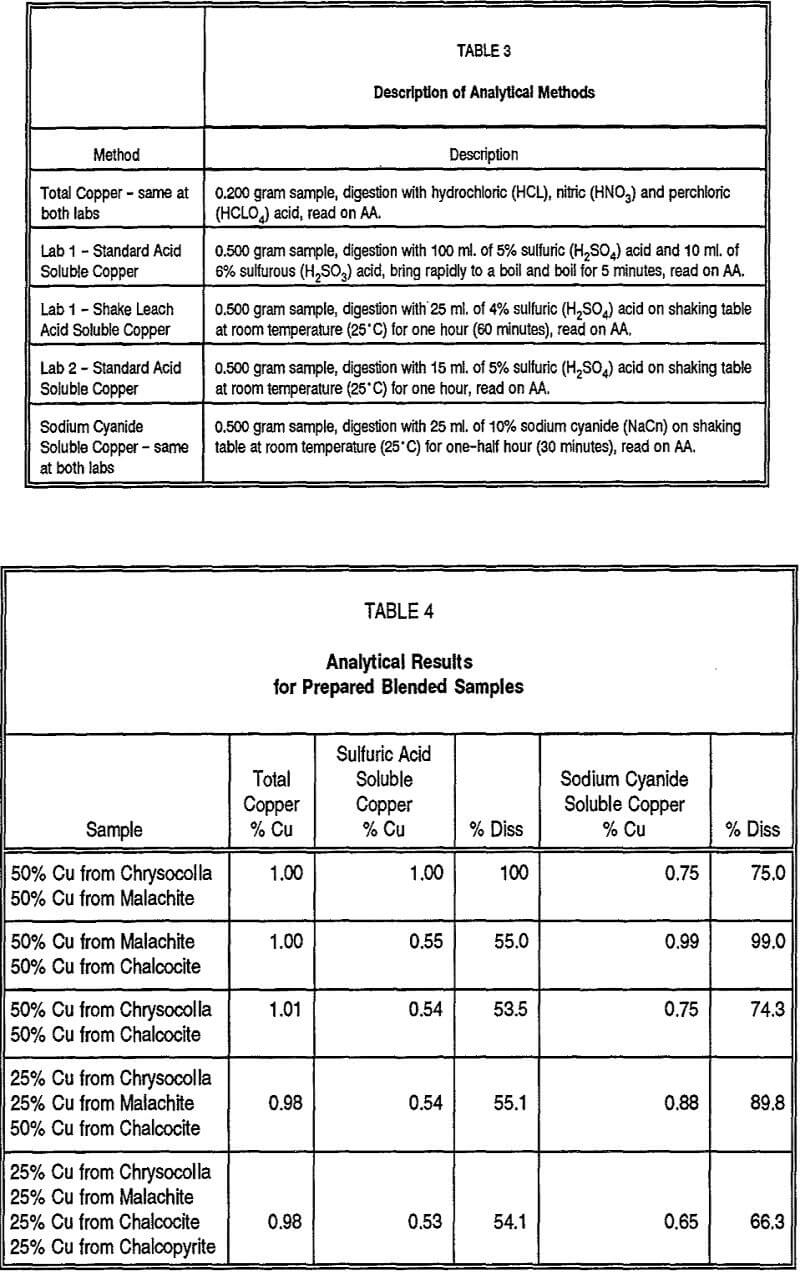
Because of the different dissolution characteristics of the oxide, secondary sulfide, and primary sulfide minerals to the different partial extraction copper laboratory analytical methods (sulfuric acid and sodium cyanide soluble copper), it is possible, by first subjecting the assay sample to sulfuric acid digestion followed by the sodium cyanide dissolution and then analyzing the sample residue for total copper in a sequential fashion, that one can selectively leach or analyze for the different mineral types present in a particular sample and effectively model the sample’s metallurgical behavior. The shake leach sulfuric acid soluble assay method attacks and quite thoroughly dissolves nearly all of the significant copper oxide minerals present, which are also amenable to standard sulfuric acid leaching conditions. If the same samples are then subjected to a sodium cyanide soluble assay, the secondary sulfide minerals and bornite would be dissolved and would represent that proportion of the sample which would be amenable to ferric-iron/sulfuric acid leaching methods. A total copper assay of the analytical residue following the sulfuric acid and cyanide dissolution steps will determine the amount of copper not dissolved by these two laboratory extraction methods and, by analogy, the proportion of copper in the sample not amenable to relatively rapid leaching. This residue will contain insoluble primary copper sulfide minerals, primarily chalcopyrite, which requires bacteriological activity and a much longer time frame to recover through leaching.
To test the sequential assay method and its ability to semi-quantitatively determine the mineralogical types present in a given sample, the same set of blended-prepared samples utilized in Table 4 were analyzed using the sequential assay method with the results presented in Table 5. With a total copper analysis of approximately one percent for each of the five samples, the results for each analytical step behaved fairly close to what was predicted based on the results shown in Table 1. The slight solubility of chalcocite in sulfuric acid is demonstrated, as is the near total insolubility of chalcopyrite, which is reflected in the residue total copper analysis. Furthermore, the summed total of the acid soluble, cyanide soluble, and residual assays closely matched the beginning total copper analysis, providing a check on the reproducibility and accuracy of the sequential assay steps.
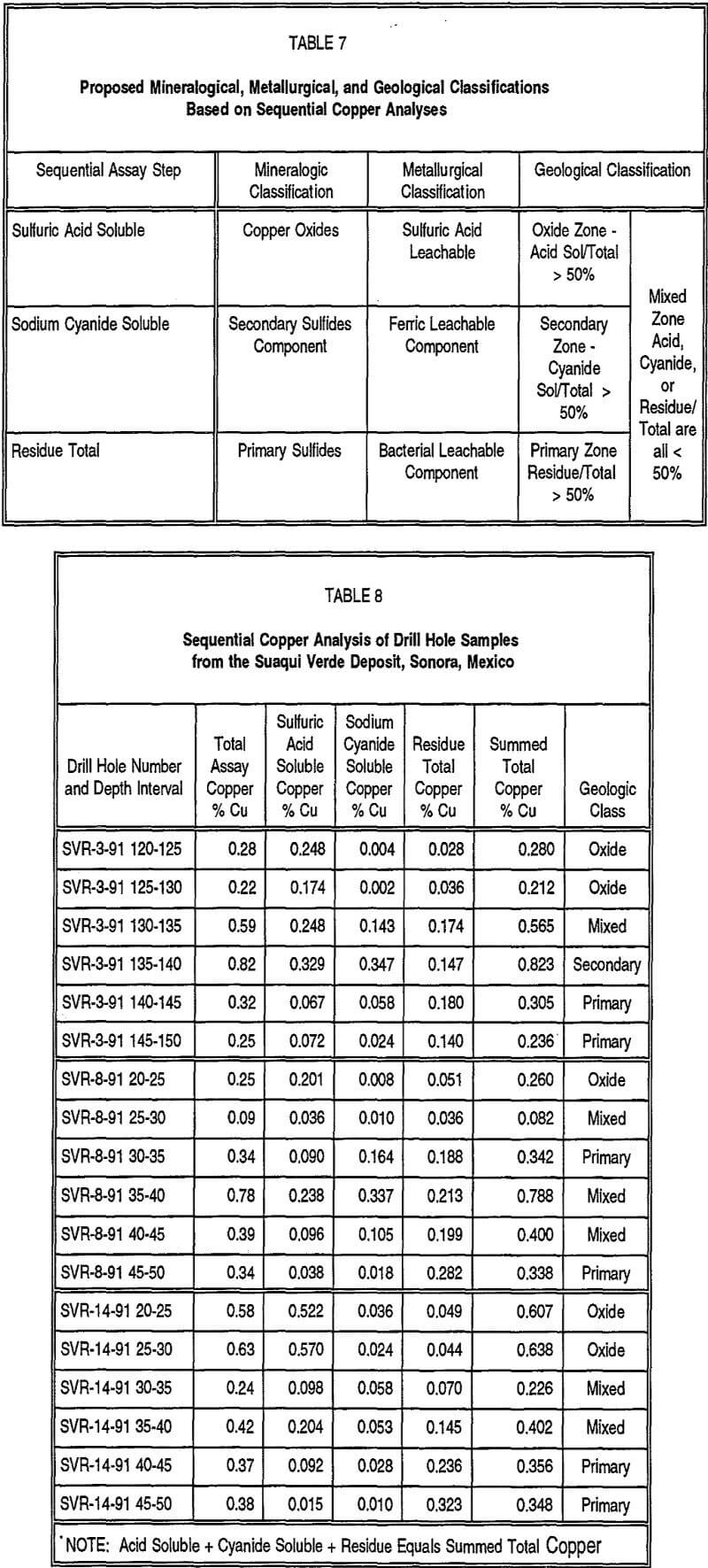
A proposed geological, mineralogical, and metallurgical classification scheme based on the results obtained from the sequential copper analysis method is presented in Table 7. This table provides a summary of the information presented earlier and suggests a method for the semi-quantitative definition of geological zones often recognized in various copper deposits. This geological classification is based on the ratios of the soluble and residual copper assays to that of the total copper assay, utilizing results from the four-step sequential copper analysis method.
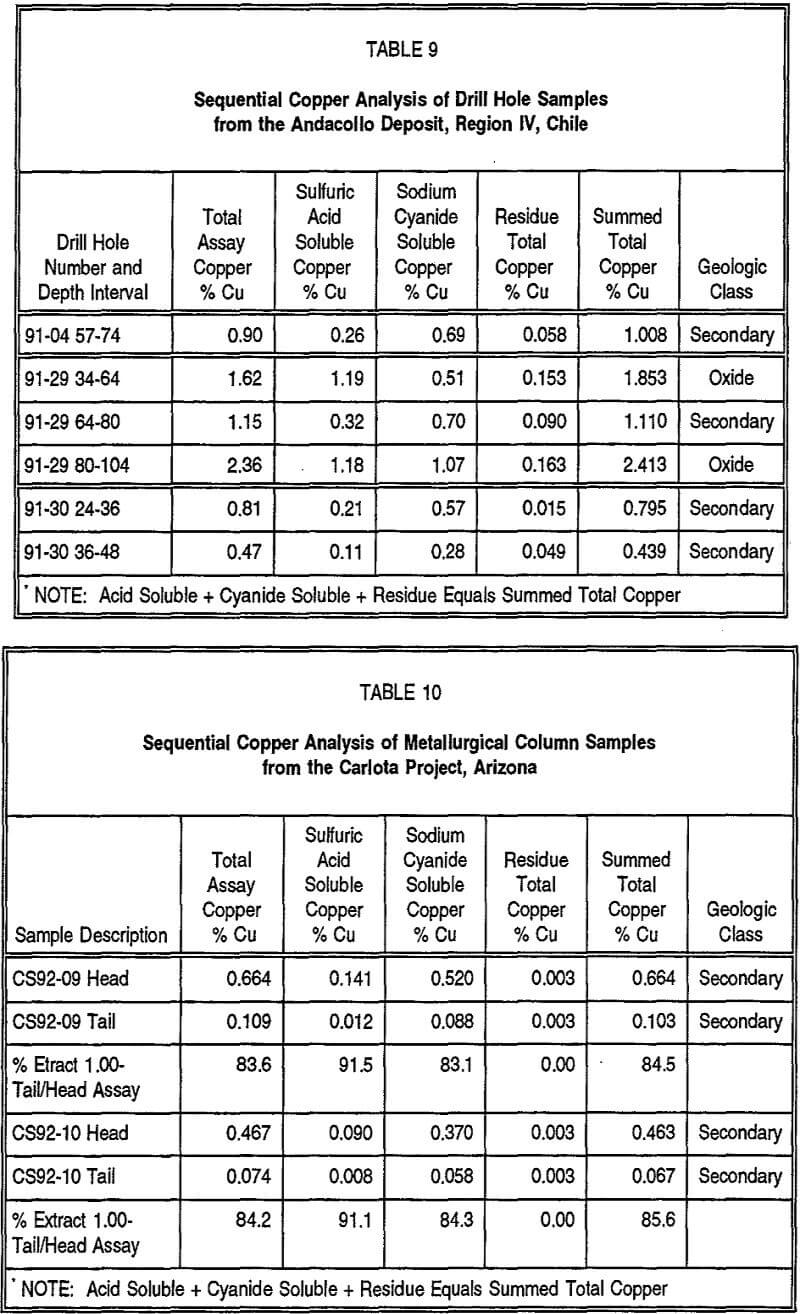
To further test the validity and applicability of the sequential assay method, assay pulps from selected drill holes from the Suaqui Verde copper deposit in Sonora, Mexico, and the Andacollo copper deposit, Region IV, Chile, were tested, with the results presented as Tables 8 and 9, respectively. Also presented in these tables is the proposed geological classification of these samples based on the sequential copper assays. Both of these deposits were difficult to evaluate for their teachable copper resources because they contain a significant portion of their mineralization within leachable secondary sulfide copper minerals, primarily chalcocite. Using only conventional total copper and oxide or acid soluble copper analyses from the drill samples, it was not possible, in many cases, to accurately portray or classify the different mineralogical or geological zones present in these deposits because leachable sulfide minerals were not distinguished from non-leachable primary sulfide minerals. In both of these deposits, it was also difficult to consistently and accurately define the mineral zones by visual examination or logging of drill core or cuttings. For the drill samples analyzed in Tables 8 and 9, there appears to be relatively good definition of the different geological zones, as well as good correlation between the total and summed total copper contents. Duplicate analyses have indicated that total copper analyses are generally reproducible to close tolerances, generally ±5 percent. However, duplicate sulfuric acid and sodium cyanide soluble analyses suggest they are reproducible to a lesser degree, typically ±15 percent, and hence, the results from the sequential copper method should be considered semi-quantitative.
The sequential assay method can also be used to assist in the definition of a metallurgical test program, as well as evaluating test results. Being able to define the various metallurgical and mineralogical types present, as well as their relative overall proportions within the deposit, allows for the selection of more accurate representative samples for metallurgical testing. Sequential copper analyses performed on pre-leaching head and post-leaching tail samples from metallurgical test work can provide valuable information relating to the percent extraction or leaching efficiency of each of the mineralogical types present in the sample and aid in selection of the most appropriate leaching parameters.
As an example, Table 10 presents analytical information on head and tail samples from two metallurgical columns from the Carlota copper deposit in Arizona. The assay results indicate that chalcocite is the predominant copper-bearing mineral in the two samples and was quite thoroughly leached during the duration of the tests, which were conducted using a ferric iron/sulfuric acid reagent.