Batch Autoclave Laboratory Procedures: The vessel used for the laboratory pressure oxidation process was a two-liter Parr titanium autoclave with dual impellers. It was heated and cooled externally and was pressurized with commercial-grade oxygen. A positive bleed of offgas was maintained in each test; the rate was measured in the offgas after the steam was removed with a condenser and desiccant. An oxygen overpressure of 100 psig (390 Kpa) was the normal operating target.
The standard procedures used for all these tests are described here. The ore was wet ground to the target size using a laboratory ball mill, and the slurry was adjusted to the desired percentage of solids. Next, the slurry was conditioned using concentrated sulfuric acid, and then autoclaved for the required time at the chosen temperature.
![]()
At the end of the preoxidation period, the autoclave was cooled, and the autoclave solids were grab sampled. The remaining portion of the slurry was neutralized and then a 24-hour carbon-in-leach (CIL) cyanidation was conducted under standard conditions.
The final leach slurry was screened to remove the carbon, which was then dried and fire assayed in total for gold. Next, each slurry was filtered and the solids were washed, dried, and fire assayed for gold. The filtrate and wash solutions were combined; a sample was assayed for gold using atomic absorption spectroscopy (AAS) after an organic extraction. From these data, the gold extraction was calculated. Other test data included sulfide oxidations, reagent additions, the concentrations of ferrous and ferric iron, and free acid levels in the autoclave slurry.
Semi-continuous Autoclave Laboratory Procedures: Figure 1 shows the configuration of the semi-continuous test apparatus. The semicontinuous procedure started with a normal batch test in order to provide a starting slurry. Next, the semicontinuous mode was commenced, in which a specified amount of slurry was discharged from the autoclave and replaced with fresh feed. Each sequence was called a cycle and was repeated several times until the system reached equilibrium. Generally, this required three to four displacements (turnovers) of the autoclave volume. In the cited work, a single test required five to six hours of operation to achieve satisfactory equilibrium. Each turnover of slurry was weighed, then combined into groups of four in preparation for CIL cyanidation. Samples of each leach composite were collected for analyses, and the remaining portion was neutralized and leached using standard CIL procedures.
Continuous Autoclave Pilot Plant Procedures: The general flow sheet started with the ore being wet ground to the target particle size, thickened, and then fed to a four-compartment continuous autoclave for preoxidation. In some cases, sulfuric acid was added to the feed prior to autoclaving. Oxygen was injected into the slurry in each compartment to oxidize the sulfides. The autoclave slurry was discharged through an automatic letdown system, then pumped into a small holding tank and sampled. Neutralization was then accomplished using two stages of pH adjustment in a continuous pilot plant circuit. The neutralized solids were sampled and the gold extractions were evaluated using standard CIL conditions in laboratory batch tests.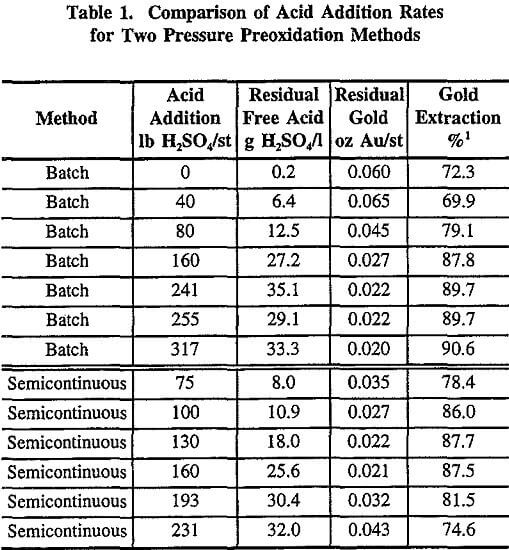
Acid Addition Levels – Batch Versus Semicontinuous
Prior to the pilot plant operation, several batch tests were conducted to determine the amount of sulfuric acid required for addition to the feed using a standard ore composite. The test composite contained significant amounts of carbonate mineralization which required the addition of acid prior to preoxidation. Levels from 0 to 317 lb H2SO4/st of feed (0 to 159 kg H2SO4/mt) were examined, using standard autoclave and cyanidation conditions. Table 1 shows that the gold extractions varied from 69.9 to 90.6%, with corresponding residual gold values of 0.065 to 0.020 oz Au/st (2.2 to 0.69 g Au/mt). The general trend was that an increase in the amount of acid produced a decrease in the residual gold content, as seen in Figure 2. An acid addition of 240 lb H2SO4/st (120 kg H2SO4/mt) appeared to be the minimum required to achieve acceptable gold extractions with residual gold values of 0.022 oz Au/st (0.75 g Au/mt). This was a rather high amount of acid and could not be accounted for by the stoichiometry of the assumed chemical reactions. It was evident that the preoxidation chemistry resulted in a higher acid consumption than predicted.