Table of Contents
This report does not constitute a finished investigation. Its purpose is to report on the status of current reduction studies and to demonstrate that high-purity ductile yttrium metal can be prepared by metallic reduction of yttrium chloride. Although both lithium and sodium have proved to be effective reductants, no conclusions can be reached on which is more favorable. The use of lithium yields a higher recovery and allows simplified operation. Sodium, however, is cheaper, is readily available in high-purity form, and can be purified during the reduction step by distillation.
More work is necessary to determine the most suitable method for preparing yttrium chloride. Although dehydration of hydrated chloride yields a high-purity product, the excessive length of time for dehydration makes this method less desirable for plant-scale use.
Considerably more work is needed before this process can be applied economically as a production operation; however, the investigation has demonstrated that pure yttrium metal is workable and that it may have potential use as an engineering material.
This investigation was undertaken to study new methods for preparing metallic yttrium in high-purity form. Yttrium is of interest because it is one of the more plentiful elements that has not been prepared commercially in the pure state. Before its true properties can be measured and before its possible applications can be determined, a method must be developed for preparing yttrium in reasonably pure form.
This program was carried out under contract with the AEC at the Albany (Oreg.) Metallurgy Research Center of the Bureau of Mines. The project was conducted as a small-scale laboratory study of various basically new procedures for preparing elemental yttrium.
Yttrium is an element that is always found associated in nature with the rare-earth elements. Although yttrium is not a member of the rare-earth group, it generally occurs with the heavier rare-earth elements because of its similar chemical properties; it is because of this close similarity that yttrium is comparatively unknown as a metal. Pure yttrium compounds did not become available for study until methods were devised at the Ames Laboratory for separating the rare-earth elements by ion-exchange techniques.
Although very pure yttrium oxide recently has become available in large quantities, this element has resisted the efforts of metallurgists to convert it into high-purity metallic form. Yttrium has a strong affinity for dissolving and retaining oxygen and nitrogen which make the element brittle and unsuitable as an engineering metal. Even when yttrium contains oxygen at no higher level than that usually associated with zirconium and titanium, it is too brittle for fabrication into useful shapes by the usual mechanical methods of forming.
Because of the extreme sensitivity of yttrium to impurities, emphasis was placed on processing methods favoring the preparation of very pure intermediate yttrium compounds and on procedures preventing recontamination during subsequent processing. Although refined techniques were employed in this laboratory study, primary consideration was given to methods and procedures that are adaptable to being expanded to plant-scale operation.
The metallic reduction of yttrium halides was chosen for study because halide salts can be prepared free of oxygen and nitrogen compounds. Although all halide salts were considered as intermediate compounds, only yttrium chloride will be covered in this report. Investigations involving yttrium bromide and yttrium iodide will be reported separately. Processes involving the metallic reduction of yttrium fluoride are not being considered in this study because of the vast amount of work already done on this approach by workers at the Ames Laboratory at Iowa State College.
Theoretical Considerations
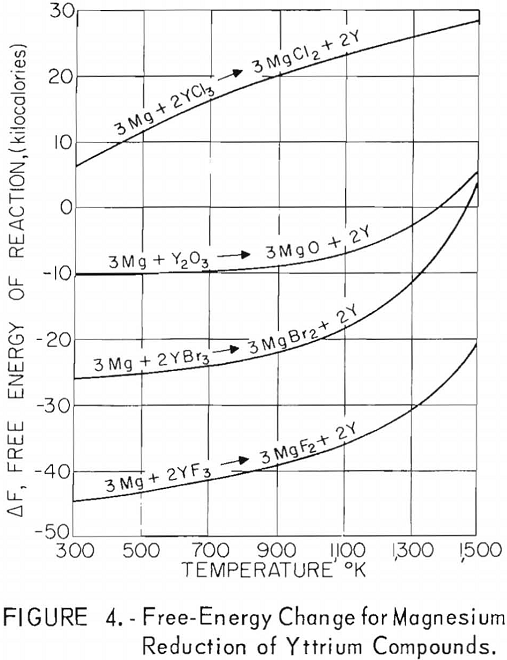
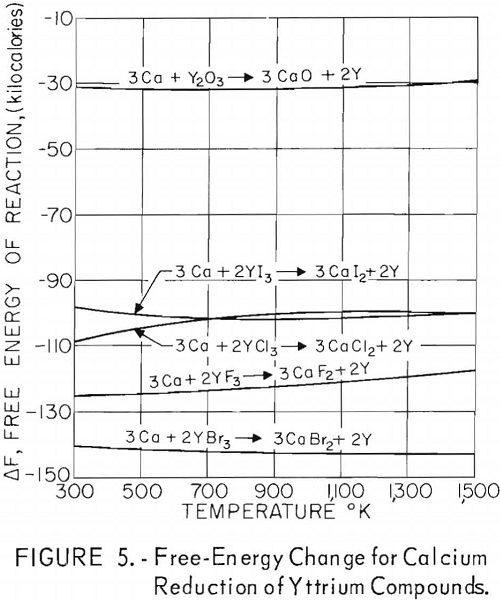
A thermodynamic study was made to determine which of the common alkali and alkaline-earth metals could be employed as reductants for yttrium halides. Although thermal data for many of the halide salts involved in these reactions are incomplete, calculations were made from the latest experimental information reported; if incomplete, they were estimated from the best information available.
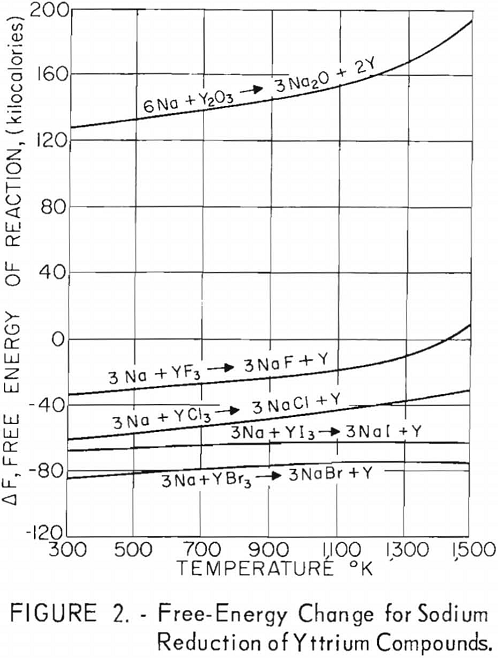
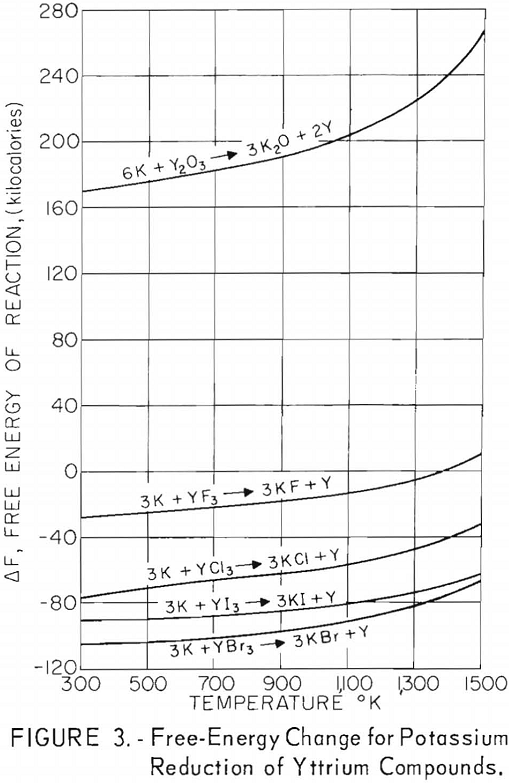
Free-energy changes for the reactions of yttrium halides with these metals are plotted against the absolute temperature in figures 1 through 5. Also shown are the free-energy changes for the metallic reduction of yttrium oxide for purposes of comparison.

These plots show that calcium should reduce all halide salts of yttrium and that magnesium might be a suitable reductant for only yttrium fluoride at lower temperatures. Of the alkali metals, only lithium should reduce all yttrium halides, and sodium should be suitable as a reductant for yttrium iodide and yttrium bromide and possibly for yttrium chloride at lower temperatures. Potassium shows promise as a reductant for yttrium iodide and yttrium bromide and possibly yttrium chloride at the lower temperatures. Although large, negative free-energy changes are indicated for many of these reactions, the fact that sodium and potassium exhibit considerable vapor pressure at the elevated temperatures necessary for operation makes it highly probable that a reverse reaction might occur during some of these reactions regardless of the indicated negative free energy. This would be true especially if operation under vacuum was contemplated.
Experimental Procedures and Results
Chlorination
Anhydrous yttrium trichloride can be prepared by several methods, but the method used repeatedly in this investigation involved the careful dehydration of hydrated yttrium trichloride. The hydrated salt was prepared by slowly dissolving yttrium oxide in concentrated hydrochloric acid. Insoluble material, primarily undissolved yttrium oxide, was separated by decantation, after which ammonium chloride was added to the solution in an amount equivalent to 4 moles per mole of yttrium. The solution was heated, and water was
evaporated until the normal boiling point reached 132° ± 1° C. At this point the solution was a thick, syrupy liquid, which upon cooling solidified to a crystalline mass containing a mixture of ammonium chloride and hydrated yttrium trichloride.
The hydrated salt was dehydrated in the nickel vacuum retort shown in figure 6. It was charged into a series of circular nickel trays to a depth
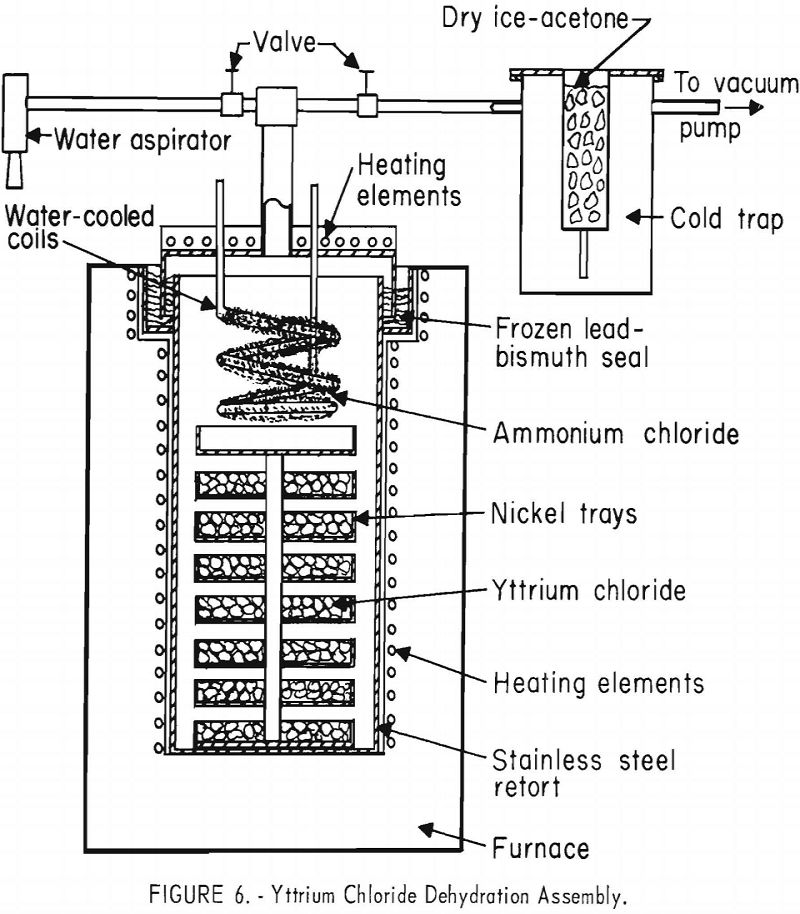
of approximately 2 inches. By heating at 100° to 120° ± 10° C. while the retort was evacuated with a water aspirator, the bulk of the water was removed from the charge. After a temperature of about 120° ± 10° C. was reached and the evolution of water had ceased, the valve leading to the water aspirator was closed, and the dehydration was continued by evacuating with a mechanical vacuum pump placed in series with a dry ice-acetone cold trap. The retort temperature was increased gradually to 350° ± 10° C. so that the internal pressure would remain within the range of 20 + 5 to 500 + 50 microns. During this period the remainder of the water was removed and condensed in the cold trap while the ammonium chloride was sublimed and condensed on the water-cooled coils suspended from the top of the retort.
By keeping the pressure at 500 microns or less during this period, the ammonium chloride was evolved slowly, maintaining an atmosphere of ammonium chloride vapors over the charge. Under these conditions the reverse reaction between water vapor and yttrium chloride was minimized, while yttrium oxy-chloride was reconverted to the trichloride by reaction with ammonium chloride.
In the apparatus depicted in figure 6, charges of 15 to 20 kilograms of anhydrous yttrium chloride were prepared in 6 or 7 days of operation. Smaller charges of about 2 kilograms were prepared in smaller scale apparatus in about 4 days. In each instance the recovery of yttrium after dehydration ranged from 95 to 98 percent of the original yttrium oxide. Although it is possible to dehydrate yttrium chloride much more rapidly by operating at higher pressures, the resulting yttrium chloride will contain much more oxychloride salt, which contributes oxygen during the subsequent reduction to metal. From yttrium trichloride prepared by careful dehydration in the manner just described, yttrium metal containing as low as 950 ± 15 p.p.m. oxygen has been prepared by lithium reduction.
Although metal of this quality is comparable to yttrium prepared by other methods, the object of this investigation was to prepare the highest purity metal possible. For this reason the anhydrous yttrium chloride usually was vacuum-distilled before reduction. This procedure not only separated most of the residual oxychloride compounds present but also converted the finely powdered yttrium chloride into a dense crystalline solid. Yttrium chloride is extremely hygroscopic, and the crystalline material after distillation presented considerably less surface to the atmosphere; consequently less hydration occurred during subsequent handling steps.
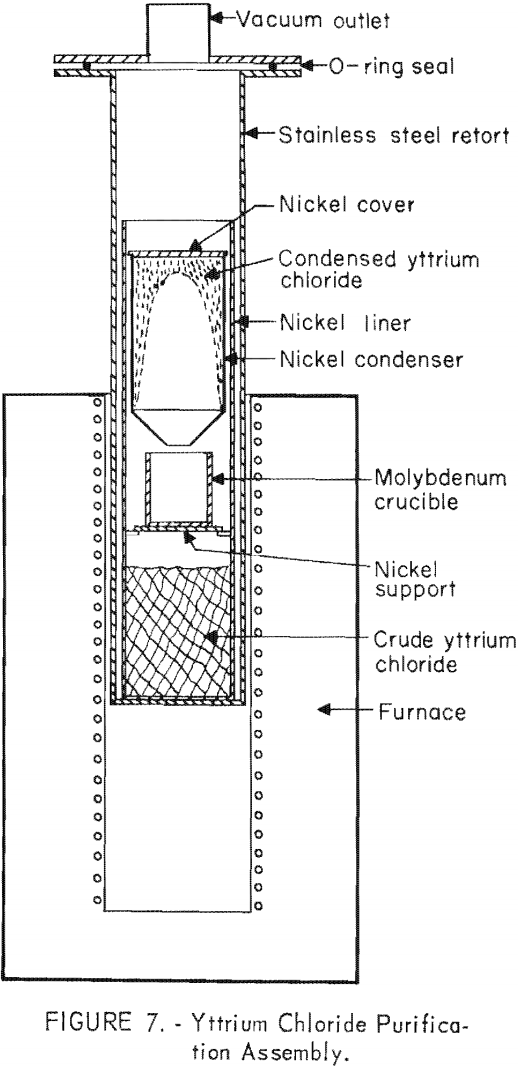
Distillation was accomplished in the apparatus shown in figure 7. The raw yttrium chloride after dehydration was charged into the nickel container shown in the bottom of the retort. A dynamic vacuum was applied to the retort to maintain the pressure between 0.4 ± 0.1 and 0«04 ± 0.01 microns while the lower end of the retort containing the charge was heated to 850° to 950° ± 10° C.
Over a 16-hour period a charge of 1 kilogram of yttrium chloride was distilled and condensed on the conical nickel shield placed in the upper, cooler region of the retort. This section of the retort was cooled by a removable water-jacket attached to the outside of the retort. Next the retort was filled with argon, the water-jacket was removed, and the position of the furnace was raised to allow the upper section of the retort to be heated to 800° ± 30° C. for about 1 hour. This melted the yttrium chloride, and it would run down into the molybdenum crucible positioned midway between the charge container and the condensing section.
By this means the yttrium chloride was cast directly into the crucible that would be used later in the reduction step. This procedure minimized handling of the yttrium chloride and exposed the least amount of surface to the atmosphere. When the chloride contained an unusually large amount of impurities, it usually was given a double distillation, because some entrainment of solid particles always occurred during distillation. In this instance the reduction crucible was not placed in the retort during the first distillation step; instead, the chloride was chipped off the nickel condensing shield and replaced in the lower container for the second distillation step. Usually 90 to 95 percent of the yttrium charged to the retort was recovered in the purified product after vacuum distillation.
The vacuum-distillation operation was satisfactory for purifying yttrium chloride, but other more direct methods for preparing yttrium chloride were investigated. Although it was felt that faster, more direct methods of making the chloride would yield a product of less purity, it was believed that vacuum distillation might be an adequate method for purification.
A method was investigated that involved the chlorination of yttrium oxide with a mixture of chlorine and carbon tetrachloride while in the presence of carbon. Yttrium oxide was mixed with an excess of carbon and a small amount of dextrin and formed into pellets or nodules. The pellets were charged into the apparatus shown in figure 8 and were heated to 600° ± 30° C. in the absence of air to decompose the dextrin. The temperature then was increased to 650° ± 30° C. while a mixture of chlorine and carbon tetrachloride was passed through the charge. Under these conditions, yttrium chloride was formed at a temperature below its melting point. To separate it from the excess carbon and unreacted oxide, the reaction product was vacuum distilled. A second distillation was required to remove carbon to a level of less than 200 ± 10 p.p.m. The overall yttrium recovery in this instance was 90 ± 3 percent.
Another effective purification procedure, especially for the separation of carbon, involved filtration of molten yttrium chloride. The impure chloride salt was placed inside a retort upon a tightly-packed pad of molybdenum wool, and a molybdenum crucible was placed in the bottom of the retort. The retort was filled with argon and heated to 800° ± 10° C. to allow the yttrium chloride to melt, pass through the filter, and collect in the crucible below. This procedure was more effective than vacuum distillation for separating gross contamination by carbon or yttrium oxide, because these solid impurities tend to be entrained in the gas stream during vacuum distillation and require repeated redistillation for complete removal. Distillation, however, was more effective in removing lower level impurities.
Another method used for preparing yttrium chloride utilized the reaction of yttrium oxalate with carbon tetrachloride. Yttrium oxalate was dried at 300° ± 10° C. for 8 hours in the apparatus shown in figure 8 and then was heated to 575° ± 10° C. while carbon tetrachloride was passed up through the charge. Chlorine was used as a carrier gas to sweep the carbon tetrachloride through the tube. It was necessary to vacuum-distill the resulting yttrium chloride to separate it from the unreacted yttrium compounds; however, no residual carbon remained from this reaction. Although overall yttrium recoveries of only 75 ± 3 percent were obtained by this procedure, it is believed that improved operating techniques would produce higher recoveries.
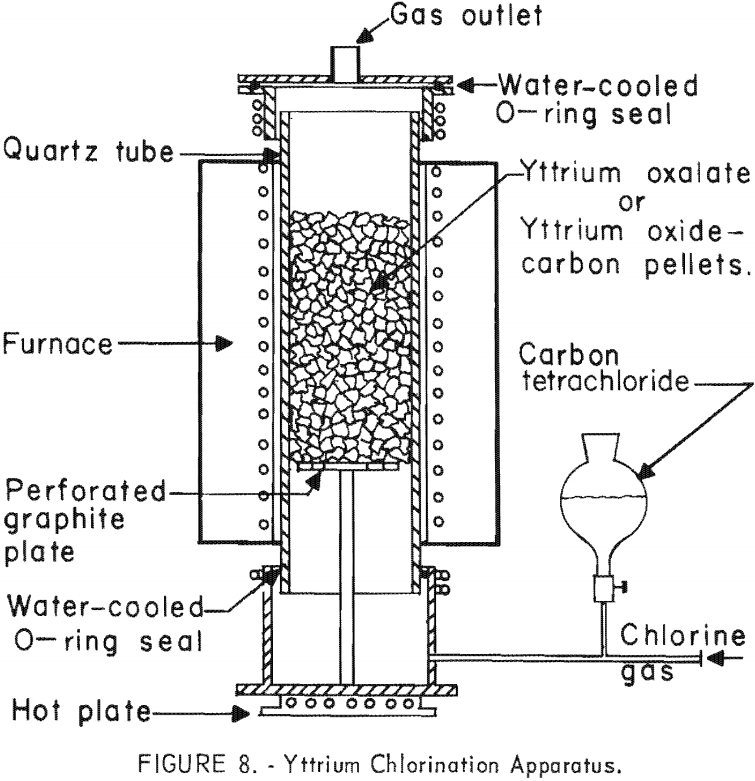
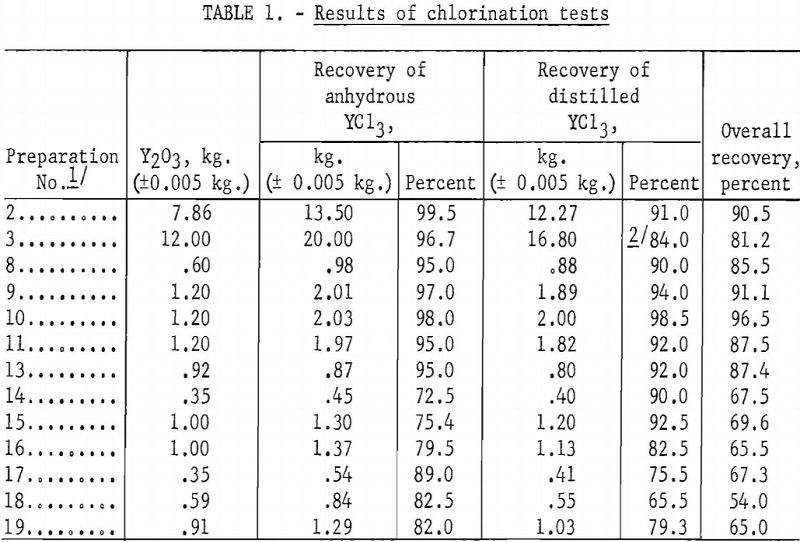
Results of chlorination tests covering these three methods of preparation are shown in table 1.
Reduction
Lithium is an effective reductant for yttrium chloride, but unless the lithium is free of impurities it will contaminate the yttrium product. The lithium used in this investigation was purchased from a commercial source in as high a state of purity as obtainable. It was received in metal containers that had been sealed under argon. The manufacturer’s analysis of the lithium used in this work follows:
Lithium analysis
Because both lithium and yttrium chloride will react with the moisture in the atmosphere, handling operations, insofar as practicable, were performed in an inert-atmosphere glove box, such as the one shown in figure 9. The lithium
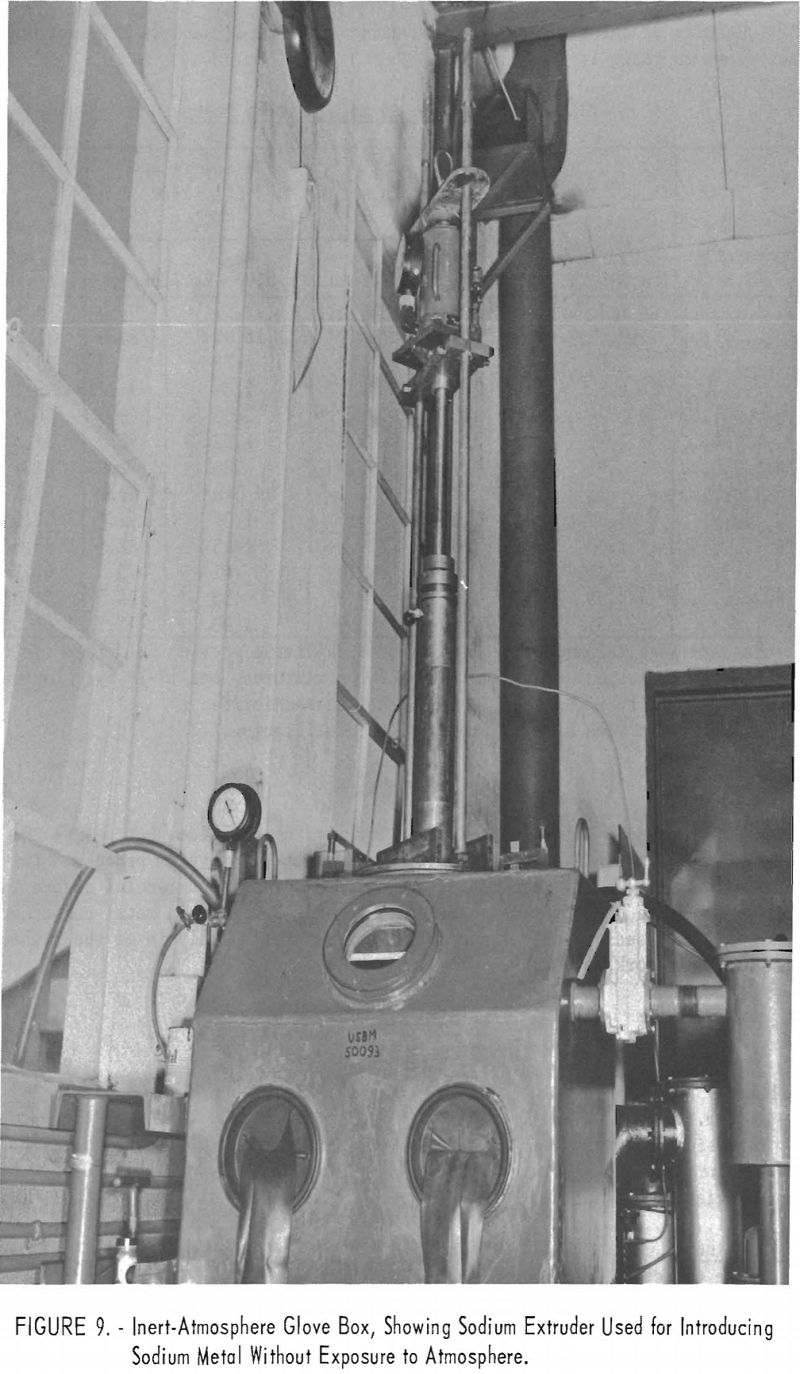
was removed from its container, and the thin, discolored outer layer was peeled from its surface with a knife.
A quantity of lithium, usually 15 to 20 percent more than the stoichiometric amount, was added to the molybdenum crucible containing yttrium chloride. The loaded crucible was transferred as rapidly as possible to the stainless steel vacuum retort shown in figure 10. The retort was evacuated to a pressure of 0.1 ± 0.02 microns by an oil-diffusion pump in series with a mechanical forepump and was then filled to 10 ± 1 inches of mercury pressure with argon. After heating at 850° ± 20° C. for 1 hour to complete the reaction, the retort was evacuated again while it was heated to 900° ± 20° C. for 16 hours to remove the excess lithium and lithium chloride from the yttrium sponge. Charges of up to 1 kilogram of yttrium chloride were reduced in this way with recoveries ranging between 95 and 100 percent.
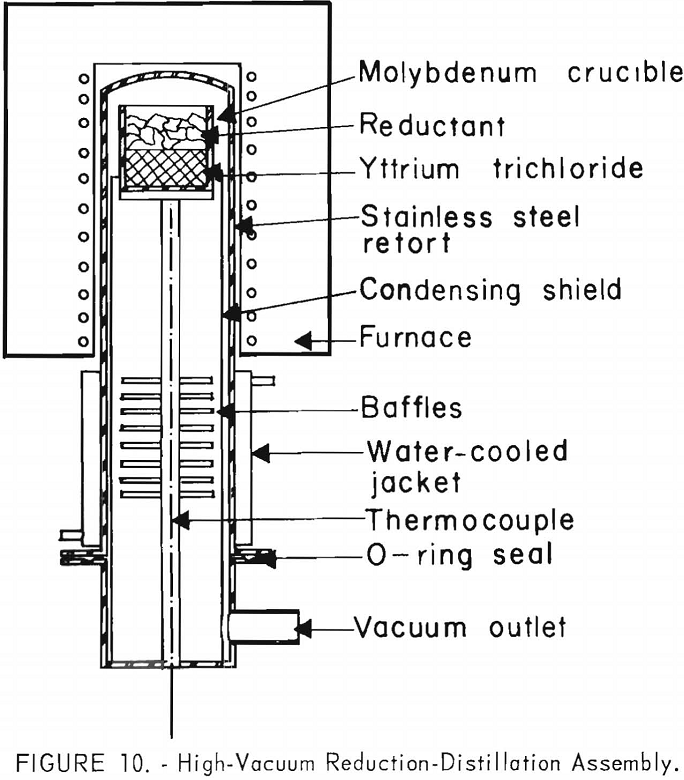
This procedure also was tested in apparatus capable of preparing up to 9 kilograms of yttrium sponge. Photographs of the equipment are shown in figures 11 and 12. Twenty kilograms of yttrium chloride from the dehydration step were charged into the vertical retort shown in figure 11. It was vacuum distilled by heating the retort for 55 hours at 950° ± 20° C. The purified chloride was reduced with lithium in the vertical retort shown in figure 12. A temperature of 900° ± 20° C. was applied for 4-¾ hours to complete the reaction. With this apparatus it was expedient to invert the crucible containing the reaction products before the byproducts were removed by vacuum distillation. This allowed the bulk of the excess lithium and lithium chloride to melt and to run out of the crucible, thereby minimizing the time required for vacuum distillation. A temperature of 950° ± 20° C. for 24 hours was required to complete the distillation. Figure 13 shows the reduction crucible containing yttrium sponge after salt removal.



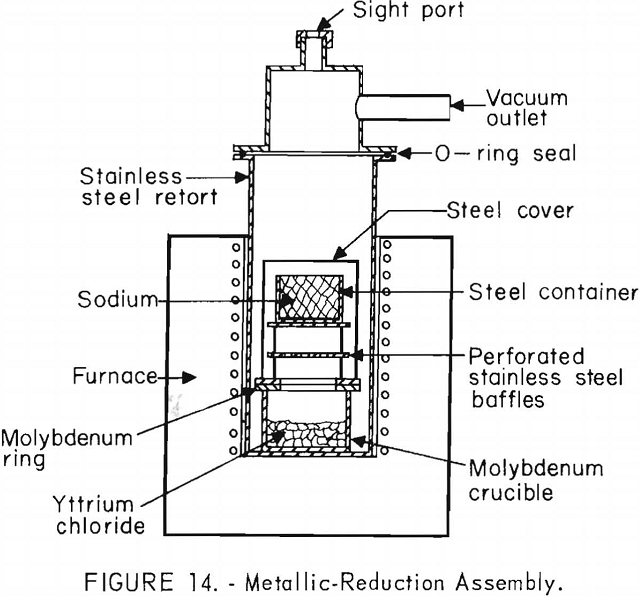
Yttrium chloride also was reduced with sodium on a scale of up to 0.7 kilograms of yttrium chloride in the apparatus depicted in figure 14. Purified yttrium chloride was charged into a molybdenum crucible in the bottom of the stainless steel retort, and an excess of sodium was placed in the steel container in the upper section of the retort.
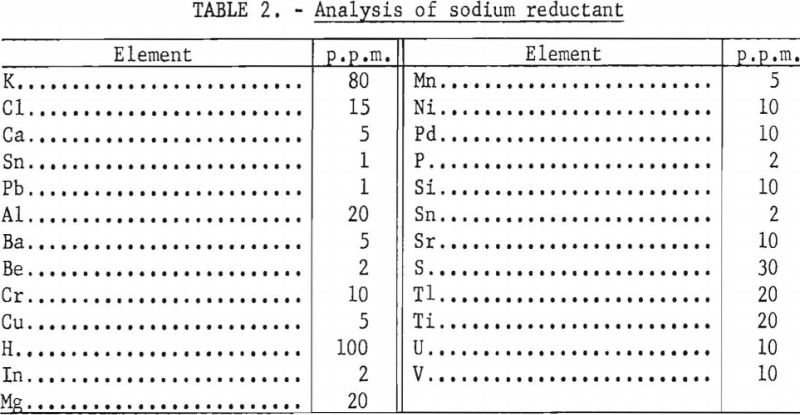
High-purity reactor-grade sodium having the manufacturer’s analysis (shown in table 2) was used for these tests. It was purchased in sealed 55-gallon steel containers. To maintain a low level of oxygen in the sodium, it was stored in the drums as a liquid a few degrees above its melting point. At this temperature sodium oxide has a very low solubility and will settle to the bottom of the drum. By removing the sodium as a liquid while under an argon cover, contamination by moisture or oxygen from the atmosphere was avoided.
After the reduction retort was evacuated and filled with argon to between 1 and 2 p.s.i. above atmospheric pressure, the lower section of the retort was heated to 850° ± 10° C. to melt the yttrium chloride. The section of the retort containing the sodium was then heated at a temperature high enough to vaporize the sodium, allowing the sodium vapors to react with the yttrium chloride until the reaction was complete. This procedure corresponds in principle to the Kroll process for producing zirconium and has the advantage of combining a distillation-purification of sodium with a means for controlling the reaction.
After the reaction was complete, the byproduct sodium chloride and excess sodium could not be separated from the yttrium by vacuum distillation. This procedure led to a reversible reaction in which yttrium reduced the sodium chloride, forming volatile sodium, which condensed and caused the reverse reaction to continue. To prevent this, the retort shown in figure 14 and 15 was designed to allow the entire retort and furnace assembly to be rotated. By rotating the retort slightly beyond 90° from the vertical position, the bulk of the excess sodium and sodium chloride was poured from the crucible. The retort was then evacuated, and heating continued to 850° ± 10° C. to remove the last traces of sodium and sodium chloride without danger of excessive reverse reaction.
Another method for preventing the reverse reaction, and one more adaptable to large-scale operation, was to cool the retort and invert the crucible before carrying out the vacuum separation procedure. In this way the bulk of the sodium and sodium chloride was allowed to run out of the crucible in the same manner as described for the large-scale lithium reduction.
Sodium reduction gave consistently lower yttrium recoveries than lithium reduction, usually in the range of 61 to 85 percent. Table 3 shows some typical results of lithium and sodium reduction tests.
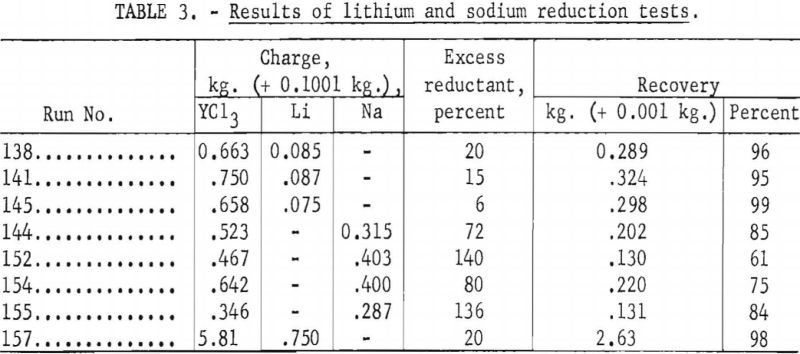
When yttrium chloride was prepared by dehydration of the hydrated chlorides, the overall recovery of yttrium as sponge usually ranged from 77 to 96 percent for lithium reductions and 61 to 85 percent for sodium reductions.
Metal Analysis
To insure homogeneity, representative specimens of yttrium sponge were consolidated into 35- to 50-gram button-sized ingots by arc-melting on a water-cooled copper hearth with a nonconsumable tungsten electrode. Hardness determinations were taken on the surface of the button after it had been dry-sanded to prepare a flat surface. Samples for analysis were taken by collecting drill shavings from several places in the button. For vacuum-fusion analysis and metallographic evaluation, solid samples were cut from the button with a hacksaw.
Yttrium metal was analyzed by semiquantitative spectrographic analysis. The method involves a moderate-current, d.-c. arc with partial sample burn. A narrow-slit, high-resolution spectrograph records the ultraviolet spectrum. Interpretation was by visual comparison to a standard plate prepared from a set of addition standards. The precision of the determination was judged to be within ± 20 percent within the range reported.
Alkali and alkaline-earth impurities were determined by an a.-c. arc method in which spectrum lines in the visible range were used. Precision was within ± 20 percent.
Oxygen was determined by vacuum-fusion analysis employing a nickel bath operated at 1,550 to 1,600° C. The precision for determining oxygen is within ± 20 percent for values below 300 p.p.m., and accuracy improves at higher concentrations.
Because nitrogen determinations performed by vacuum fusion analysis were unreliable, nitrogen was determined by Kjeldahl analysis. This procedure is precise to within ± 10 percent within the range of 30 to 100 p.p.m.
Carbon analyses were made by conductometric determination. Precision in this instance was within ± 5 percent within the range of 100 to 200 p.p.m.
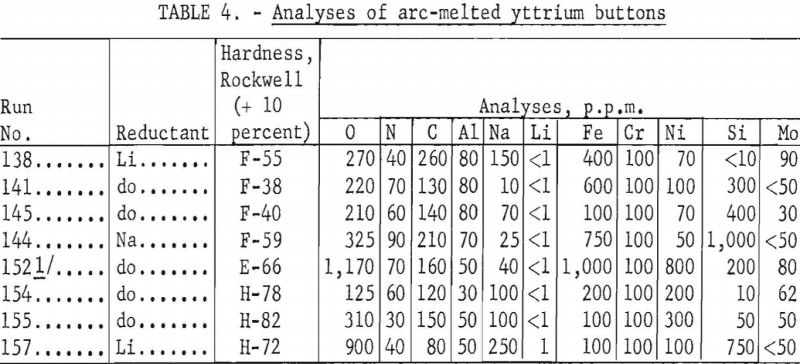
Table 4 is a listing of representative analyses of yttrium prepared by both lithium and sodium reduction. The run numbers in this table correspond to the run numbers shown in table 3. Hardness determinations also are given. It appears that the metal hardness increases with the oxygen content, as might be expected, although a valid correlation cannot be drawn because other impurities were not held constant.
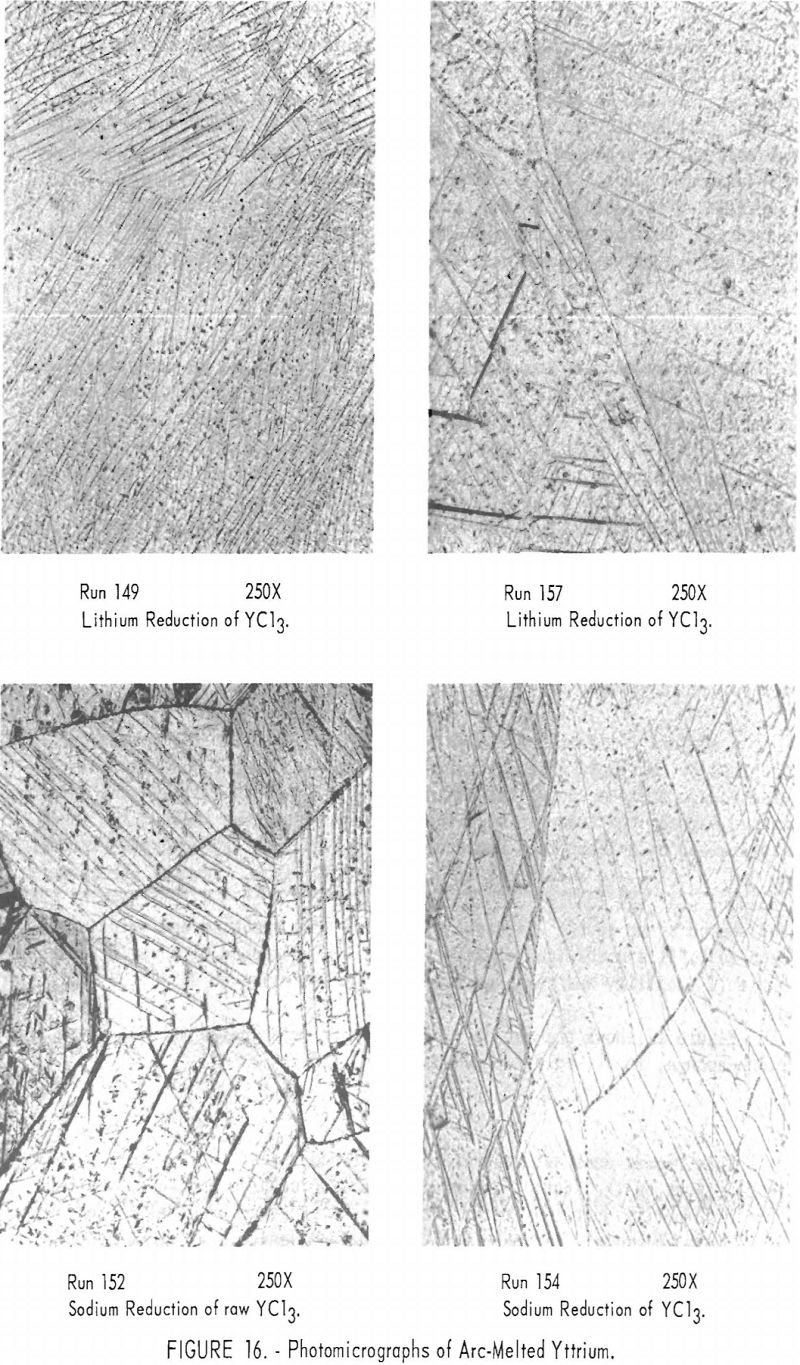
Photomicrographs of several yttrium specimens are shown in figure 16. The following metallographic preparation procedure was employed: The specimens were mounted in bakelite and polished on a canvas wheel with silicon carbide grit ranging in size from 280-mesh through minus-600-mesh; kerosene was used as the vehicle. Final polishing was accomplished with minus-50,000-mesh diamond powder. The specimens were etched for 1 or 2 seconds with concentrated nitric acid, rinsed with water immediately and then with alcohol, and dried. The photomicrographs show considerable twinning in the as-cast structure, which was caused when the specimens were cut and mounted.
Mechanical Working
Yttrium metal also was evaluated by cold-rolling the as-cast arc-melted buttons or sections of the button. Buttons were rolled through cold reductions as high as 98 ± 1 percent without failure, except for slight edge cracks, which did not propagate. The metal work hardened rapidly on rolling, and the sheets could not be bent unless they were first annealed by heating in a vacuum to 600° ± 30° C. for about 5 minutes. When fully annealed yttrium is bent, a distinct “cry” can be heard, very similar to that of tin.
At this stage of the investigation it has not been possible to correlate workability with the various impurities present in the metal. It appears, however, that yttrium containing over approximately 700 p.p.m. oxygen cannot be cold-rolled; the metal containing less than 400 p.p.m. usually can be severely cold-rolled.
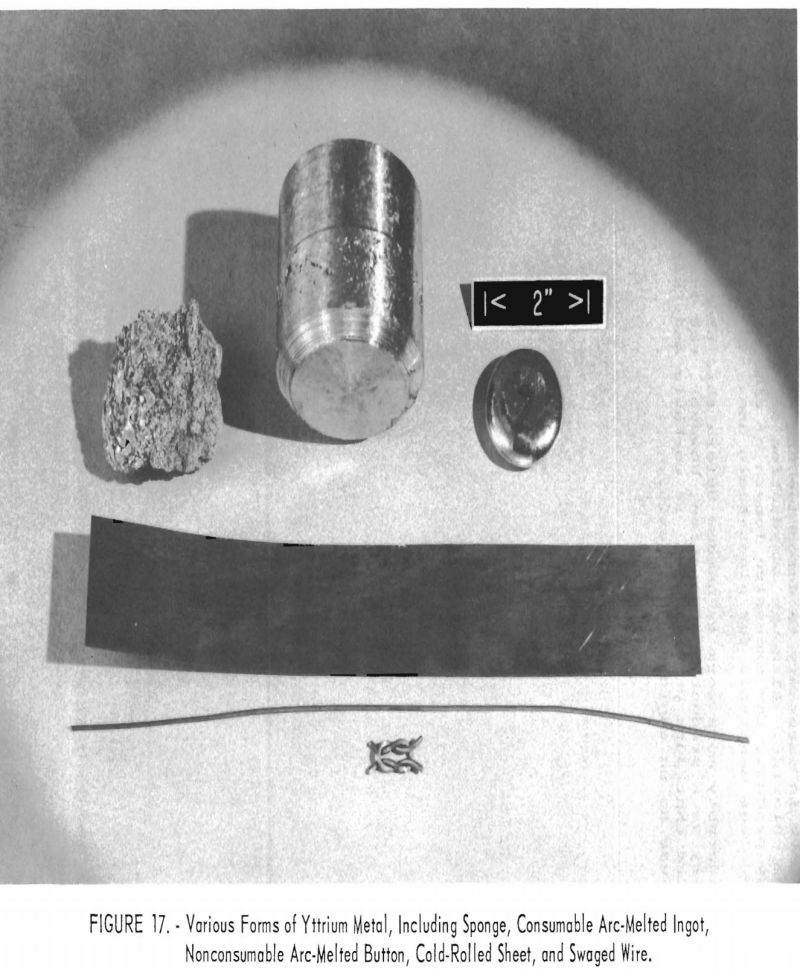
A nominal 1-½-inch diameter by 2-inch long yttrium ingot, was prepared by cold-mold arc-melting yttrium sponge, using a nonconsumable electrode. Yttrium sponge was added periodically to the molten pool maintained between the tungsten electrode and the top of the ingot. The ingot was scalped on a lathe to a diameter of 1 ± 0.05 inch and was warm-extruded at 350° ± 50° C. to a diameter of 0.3100 ± 0.005 inch. Several checks were apparent near the leading end of the extruded rod, but the remainder of the rod seemed to be sound. Sections of the rod were annealed and cold-swaged down to 0.051 ± 0.002-inch-diameter wire. The as-worked wire was brittle, but full ductility was restored after vacuum annealing at 600° ± 30° C. Figure 17 shows the various forms of yttrium prepared in this investigation—sponge, ingot, cold-rolled sheet, and wire.
