PREPARATION OF SOLUTION FOR BASES
1. Boil the finely-divided substance in distilled water.
2. If insoluble, add ¼ its bulk of strong HCl and boil for two or three minutes.
3. If still insoluble, treat a fresh portion with strong HCl and boil for five minutes ; then add an equal volume of water and warm.
The majority of substances will dissolve with the above treatment.
4. If there is still an insoluble residue, decant off the solution from (3), add a little fresh HCl, boil, then add strong HNO3, drop by drop, as long as the substance appears to dissolve (about 6 drops should suffice).
Certain sulphides and a few other compounds are dissolved by this treatment.
If no action occurs on treating thus, decant off the acid and neglect it. Wash the insoluble residue and treat by table for insolubles.
5. Insoluble double cyanides (indicated by a strongly-coloured insoluble) require special treatment by fusing in a porcelain crucible with three times their bulk of NH4NO3 and (NH4)2S11O4 (1 : 3) in a fume chamber, dissolving in HCl and treating as usual. (Ba, Sr, Pb will remain insoluble; As and Hg may be volatilized.)
6. Metals or alloys should be in a fine powder or in thin sheets.
(a) Obtain solution as usual.
(b) If solution is slow, treat with dilute HNO3 and, if still slow, with strong HNO3; dilute and filter.
Residue.—Au, Pt, SnO2, Sb2O4, Sn (as arsenates), Bi (as arsenate or phosphate). Test for Au and Pt separately in Aqua Regia solution.
Warm with (NH4)2S and filter.

If the original residue is white, probably only Sb2O4 and SnO2 are present. Boil with strong tartaric acid and filter.
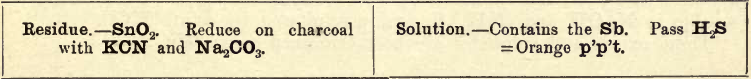
Note 1. If much HCl has been used to obtain a solution, evaporate nearly to dryness and add H2O.
Note 2. If HNO3 has been used to obtain a solution, add strong HCl and evaporate nearly to dryness to drive off HNO3 and then dilute with H2O.
Note 3. The solution should be free from HNO3 and not contain much HCl.
NOTES ON THE PREPARATION OF SOLUTION FOR BASES.
The substance is first treated with distilled water, boiled, and then allowed to cool; if it is not all soluble, some of the clear solution is decanted off and tested with HCl (bench reagent) for presence of Group I.
If Group I. is present, the whole of the water solution must be filtered off and HCl added to it ; the solution is then added to the insoluble residue, together with a few drops of strong HCl and boiled for three minutes ; if a residue still remains, the dilute HCl solution is filtered off and the residue treated with a little strong HCl. If soluble, the strong HCl solution is added to the dilute HCl solution; but if there is still a residue it is treated with fresh HCl boiled, and HNO3 added, drop by drop, as long as the substance appears to dissolve.
If HNO3 has been used to get the substance into solution, it has to be expelled before proceeding by the group tests; therefore it is advisable to keep this solution as small as possible.
If insoluble double cyanides are present in the substance to be treated, a solution in the ordinary way should be obtained and treated as far as possible, besides the solution obtained after decomposing the double cyanides.
NOTES TO THE GENERAL TABLE.
1. If Cr has been indicated in the preliminary, and the solution has a yellow or orange colour, chromates or bichromates may be present and must be reduced to chromic salts; therefore, after adding HCl, add alcohol and boil till the odour of aldehyde or alcohol is dispelled before passing H2S.
2. If cyanogen was detected in the preliminary, test original for double cyanides before proceeding in the usual way.
3 If I or Br is present the solution should be boiled with Aqua Regia before passing on to Group II.
4. If the original substance had to be dissolved in HCl and did not require the application of Notes 1, 2, or 3, H2S may be passed at once.
5. HCl will produce a p’p’t in a saturated solution of a Ba salt, soluble in hot water. From alkaline solutions HCl precipitates gelatinous H4SiO4, crystalline p’p’ts of boric, benzoic, and uric acids, also amorphous Sb2O5, metallic oxides as Al2O3, and sulphides, AS2S3, Sb2S3, Sb2S5, SnS, SnS2, (soluble in NaOH and (NH4)2S2 and precipitated by dilute HCl).
These oxides and sulphides are best examined separately.
6. If As has been detected in the preliminary, this filtrate, which may contain pentad As, should be boiled with H2SO3 and the acid solution evaporated considerably to expel SO2, Ba, Sr, or Pb, if present, may be precipitated, partially or wholly, as sulphates, and the p’p’t should be examined separately.
7. Oxychlorides of Bi, Sb, and Sn may be precipitated on the first addition of HCl (dilute) or water, but are readily dissolved in more acid on gently heating.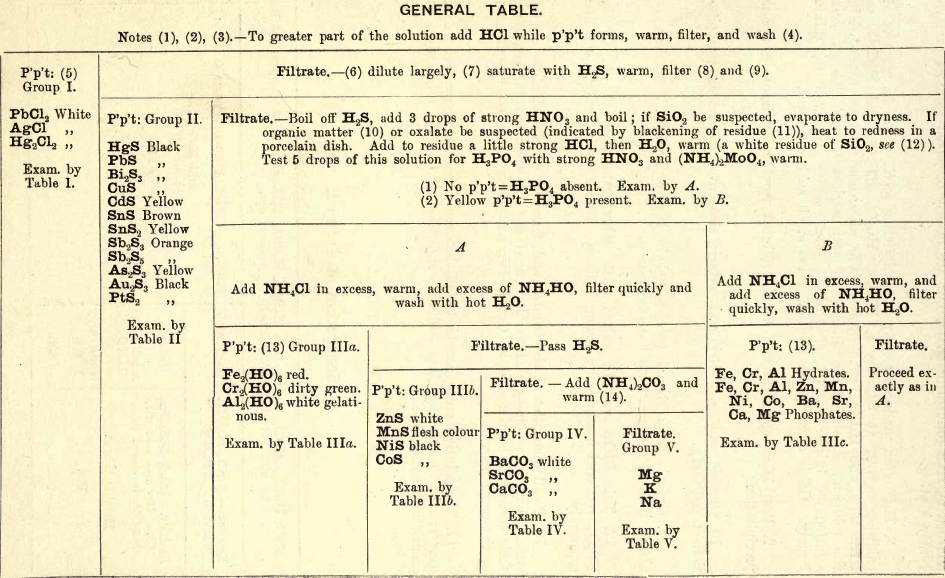
8. H2S often produces merely a precipitation of S, owing to presence of oxidising agents, as Cl, Br, I, HNO3, HClO, HBrO3, and H2CrO4 or ferric salts, also H2SO3, etc. This p’p’t is white and remains suspended in the solution; it should be filtered off and neglected. In strongly acid solutions a brick-red p’p’t of PbSCl2, often comes down; if so, dilute largely. Cd is often left in solution if too much acid is present.
9. Pass H2S again through filtrate (diluted further) to insure complete precipitation.
10. Organic acids, e.g. citric and tartaric, also sugar, prevent the precipitation of Al2(OH)6.
11. Organic acids should be detected in the preliminary. If they are absent it is not necessary to heat to redness.
12. This SiO2 may be mixed with other substances, Al2O3, Cr2O2, F2O3, rendered insoluble by strong ignition; BaSO4 and SrSO4; examine separately.
13. Small quantities of the borates and fluorides of the alkaline earthy metals may be precipitated here, but need not be examined further, since their bases will be detected in Group IV., and their acids on examination in the usual way.
14. The solution must not be boiled, because the NH4Cl present would convert the carbonates into soluble chlorides, the CO2 volatilizing as ammonium carbonate.

Note. L The NaOH should contain no Al2O3, ; if it does, test a similar amount by acidifying with HCl and adding NH4HO. Compare this p’p’t with one obtained.
Note 2. Traces of Mn may appear here, indicated by bluish green mass changing to purple in solution.
Note 1, If p’p’t is not black, no Ni or Co need be looked for. If black p’p’t passes through filter, Ni is nearly certain to be present.

Note.— Always test for NH4 in the original substance by boring with NaOH; NH3 evolved indicates NH4 present.
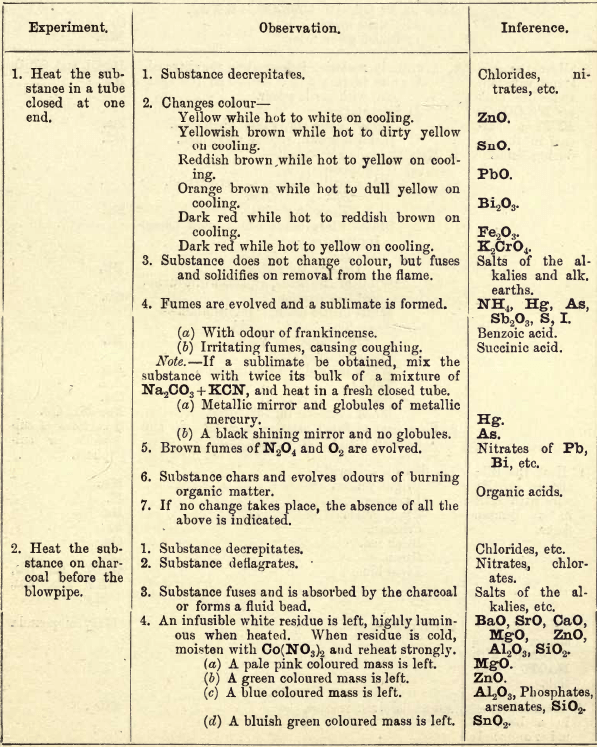
Experiment I.—In heating substances in a glass tube, closed at one end, care should be taken not to start with too high a temperature. Use a low bunsen flame and gradually raise it, noting from stage to stage the changes that take place. If the substance decrepitates—i.e., flies into smaller particles—chlorides, nitrates, etc., may be expected, and should be carefully looked for when testing for acids later on. Test any moisture that is given off with litmus paper to see if it is acid, alkaline or neutral.
When heated at a fairly high temperature, some salts part with their acids and are converted into the oxides of the bases. Observation 2 gives the changes in colour of these oxides from hot to cold, by which they can be recognised. Observation 3 indicates the presence of salts of the alkalies and alkaline earths, by not changing colour, but by fusing and solidifying when removed from the flame.
If, at a low temperature, fumes are evolved and a sublimate is formed, i.e., a condensation of the volatile substance takes place in the upper or cool part of the tube, Sb2O3, NH4, S, I, Hg, As, may be suspected.
Sb2O3 (white fumes) will only rise and settle down in the tube again.
Fumes of NH4 compounds, Hg and As and some of their compounds, generally rise right up the tube and condense on the cooler part of the tube. S and I also sublime, but can be more easily recognised by their colour—I giving violet vapours condensing to black metallic-looking plates, and S giving yellowish fumes and a yellow sublimate.
As and Hg compounds are reduced by KCN or charcoal to the metallic state and give a black mirror, Hg showing small globules when examined closely, or when the mirror is scraped together with a small splint of wood.
When two or more substances are present together, the reactions of the one may more or less mask the other. Students must be careful not to mistake the darkening of some substances for charring. It is better to make certain by heating strongly some of the original substance on a porcelain dish or crucible lid, when the result can be better seen.
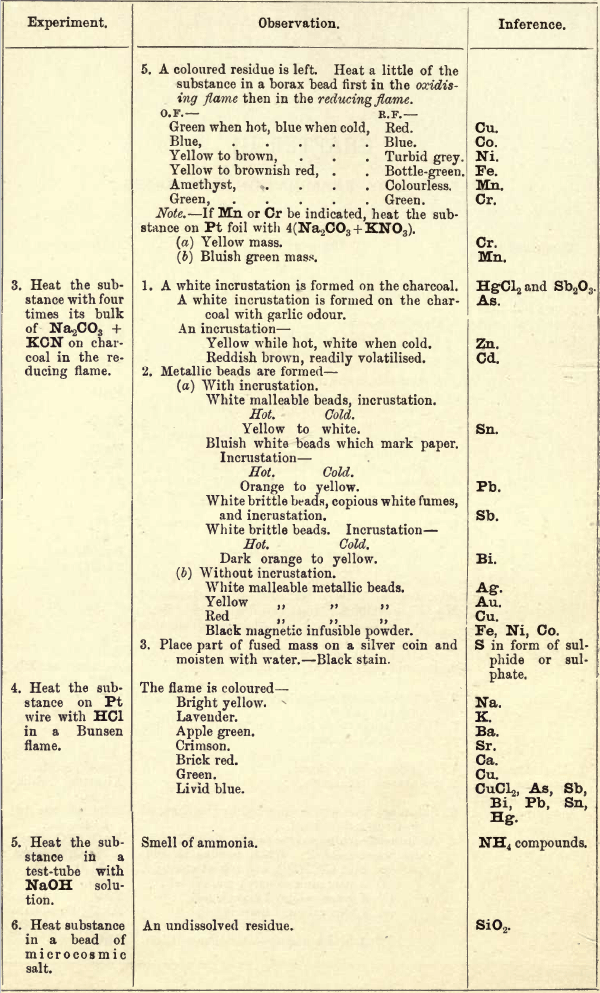
Experiment II. — On charcoal before the blowpipe, chlorides, etc., decrepitate. Chlorates and nitrates, which readily part with some of their oxygen, deflagrate. Salts of the alkalies fuse easily, so are more or less absorbed by the charcoal, and then form fluid beads. The oxides of the alkaline earths, also those of Mg, Zn, Al, Si, etc., are not easily fusible, but remain as white residues, which are highly luminous when heated. Some of these oxides, when heated with Co(NO3)2, give typical colour reactions, but the oxides of Ba, Sr and Ca remain unchanged, so can be distinguished from the others. Cu, Co, Ni, Fe, Mn, and Cr salts, when heated strongly, leave coloured residues which give typical colour reactions in a borax bead, due to the formation of borates of the metals. If two or more of these metals be present together, the lighter colours will be masked by the darker. Co generally hides the others, and with Ni the blue tint is deeper.
Cr and Mn, when fused with Na2CO3 and KNO3, form chromates and manganates of K and Na, which give the yellow and bluish-green colour to the fused mass.
Experiment III.—On charcoal, with the flux Na2CO3 and reducing agents KCN and charcoal, some metals are reduced and give beads of the metal, while others give both beads and incrustations. These incrustations are generally the oxides of the metals, formed by the different metals volatilising, and as they pass through the oxidising zone of the flame, they become oxidised and condense on the cooler part of the charcoal. The change of colours of these incrustations from hot to cold corresponds with the changes in the closed tube of the oxides of the different metals. Fe, Ni, and Co are converted into black magnetic oxides. FeS may be present in the black mass, being a fusible sulphide.
Experiment IV.—Some chlorides of the metals are volatile and give typical flame reactions; the other salts of these metals do not all give the same reactions, hence there is some difficulty in getting these reactions, especially with some sulphates, e.g.. CaSO4.
Experiment V.—NH3 must be tested for here, as ammonia salts are added to the solutions from time to time in the wet tests, so cannot be tested for there.
Experiment VI.—SiO2 can generally be detected here by the undissolved residue in the bead, but when fused for some time, some silicates leave no residue, so care must be exercised here. If minerals are to be tested, take a chip in preference to the powdered mineral.
GENERAL TABLE.
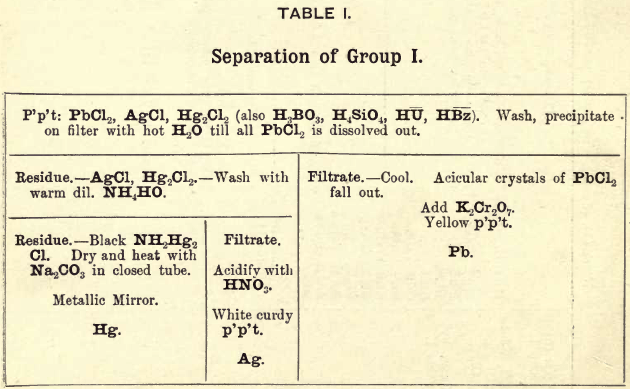
Group I. consists of chlorides of Pb, Ag, and Hg (mercurous), precipitated from an aqueous solution by the addition of HCl.
The following equations show some of the reactions:—
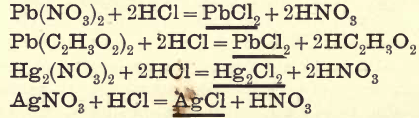
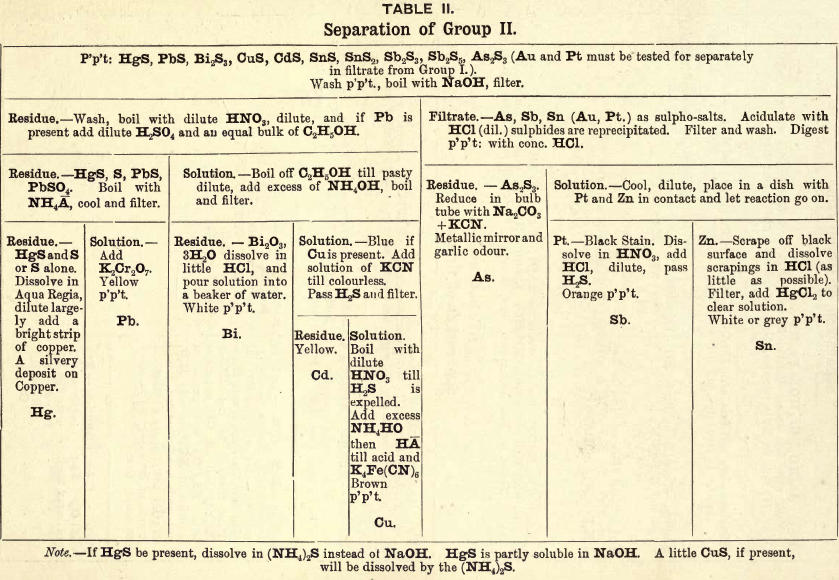
Group II. consists of sulphides of Hg, Pb, Bi, Cu, Cd, Sn, Sb, As, etc., precipitated from a dilute HCl solution by passing H2S gas slowly through the solution to saturation.
The following equations show the reactions:—
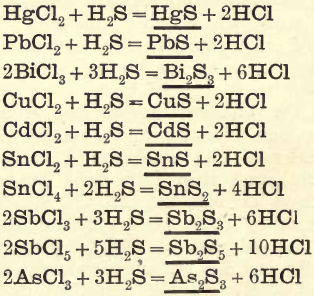
Note.—Formulae underlined represent precipitates. The arrow sign (→) represents a volatile substance.
After the precipitation of Group II., it is necessary to rid the filtrate of the excess of H2S by boiling H2S being a reducing agent, reduces any ferric salts that may be in solution to ferrous salts, and before proceeding to precipitate Group IIIa, it is necessary to reconvert them back to the ferric form in order to completely precipitate them with NH4OH : therefore, after getting rid of the reducing agent, HNO3 is added to assist oxidation.
3FeCl2 + 3HCl + HNO3 = 3FeCl3 + NO + 2H2O
Before precipitating Group IIIa, the solution is tested for H3PO4, as NH4OH precipitates the phosphates of Groups IIIb, IV., and of Mg.
The precipitate formed on the addition of (NH4)2MoO4 is believed to have the composition 12MoO3, (NH4)3PO4.
Group IIIa.—In the presence of NH4Cl the hydroxides of Mn, Zn, Ni, Co, and Mg are not precipitated by NH4OH, owing to the formation of soluble double chlorides. (For example, see Group V.)
The following equations show the reactions with NH4OH:—
Fe2Cl6 + 6NH4OH = Fe2(OH)6 + 6NH4Cl
Cr2Cl6 + 6NH4OH = Cr2(OH)6 + 6NH4Cl
Al2Cl6 + 6NH4OH = Al2(OH)2 + 6NH4Cl
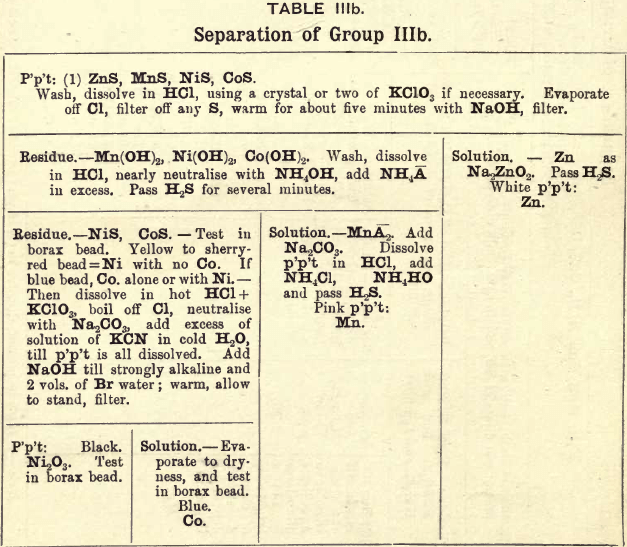
Group IIIb consists of the sulphides of Zn, Mn, Ni, and Co precipated from an ammoniacal solution by passing H2S gas slowly through it to saturation.
The following equations show the reactions :—
ZnCl2 + (NH 4)2S = ZnS + 2NH4Cl
MnCl2 + (NH4)2S = MnS + 2NH4Cl
NiCl2 + (NH4)2S = NiS + 2NH4Cl
CoCl2 + (NH4)2S = CoS + 2NH4Cl
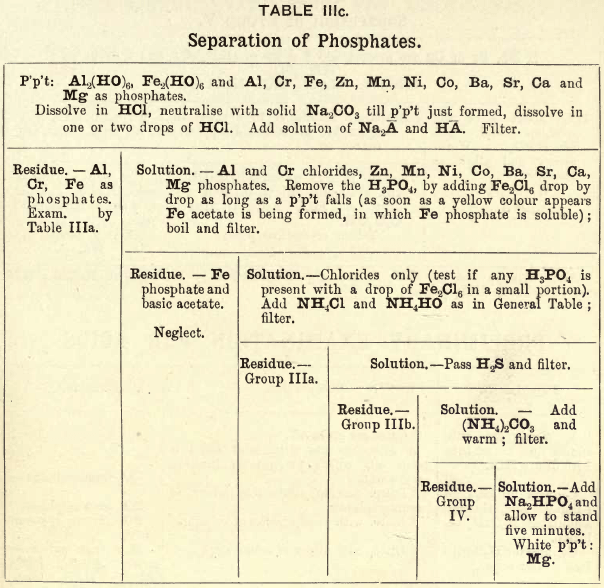
Group IIIc consists of hydrates of Fe, Al, and Cr, also phosphates of Fe, Cr, Al, Zn, Mn, Ni, Co, Ba, Sr, Ca, and Mg. All these phosphates are soluble in HCl but are precipitated by the addition of solutions of the alkalies, such as NH4OH, KOH, and NaOH. For example—
Ca3(PO4)2 + 6HCl = 3CaCl2 + 2H3PO4
BaHPO4 + 2HCl = BaCl2 + H3PO4
3CaCl2 + 2H3PO4 + 6NH4OH = Ca3(PO4)2 + 6NH4Cl + 6H2O
BaCl2 + H3PO4 + 2NaOH = BaHPO4 + 2NaCl + 2H2O
Group IV. consists of the carbonates of Ba, Sr, and Ca, precipitated from an ammoniacal solution by (NH4)2CO3.
The following equations show the reactions—
BaCl2 + (NH 4)2CO3 = BaCO3 + 2NH4Cl
SrCl2 + (NH4)2CO3 = SrCO3 + 2NH4Cl
CaCl2 + (NH4)2CO3 = CaCO3 + 2NH4Cl
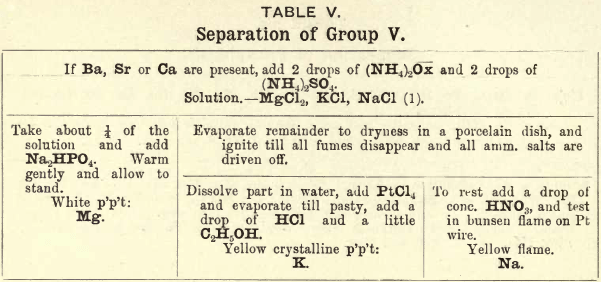
Group V.—Mg is precipitated as phosphate by the addition of Na2HPO4 to the ammoniacal solution.
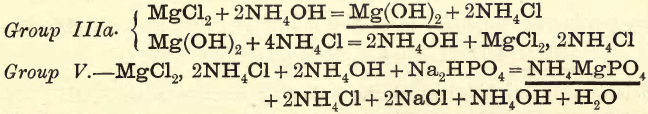
NH4MgPO4 is appreciably soluble in H2O but insoluble in NH4OH.
K is precipitated, after driving off the ammonium salts, by PtCl4. K2PtCl6 being soluble in H2O and the alkalies but insoluble in alcohol, the latter is added to completely precipitate it.
2KCl + PtCl4 = K2PtCl6
REACTIONS IN GROUP I.
In this group Ag, Pb, and Hg (mercurous) are thrown down as chlorides, advantage being taken of the insolubility of these chlorides in cold water solutions.
PbCl2 being soluble in boiling water, is separated from the other two by treating the mixed precipitates of the three bases with boiling water and filtering the solution quickly, then allowing it to cool, when the PbCl2 comes down again in acicular crystals.
AgCl is insoluble in hot water but readily soluble in NH4OH.
2AgCl + 3NH4OH = 2AgCl.3NH3 + 3H2O
This compound (2AgCl.3NH3) is decomposed by HNO3
2AgCl.3NH3 + 3HNO3 = 2AgCl + 3NH4NO3
Hg2Cl2 by the action of NH4OH is converted into the black mercurous ammonium chloride.
Hg2Cl2 + 2NH4OH = NH2Hg2Cl + NH4Cl + 2H2O
This mercurous compound, in the presence of AgCl and excess of NH4OH, passes into the corresponding and more stable mercuric salt, NH2HgCl, and the mercury it loses reduces a portion of the AgCl.
Hg2Cl2 + 2AgCl + 4NH4OH = 2NH2HgCl+2NH4Cl + 4H2O + 2Ag
In this separation a small quantity of silver, in the presence of a large amount of mercury, might not be detected, being entirely precipitated with the mercury compound.
REACTIONS IN GROUP II.
In this group certain of the sulphides are soluble in (NH4)2S, also in KOH and NaOH, which affords an opportunity of dividing it into two divisions. Division I. contains the sulphides of As, Sb, and Sn (Au and Pt) soluble in (NH4)2S, KOH, and NaOH.
As2S3 + 3(NH4)2S = 2(NH4)3AsS3
As2S3 + 6KOH = K3AsO3 + K3AsS3 + 3H2O
As2S3 + 6NaOH = Na3AsO3 + Na3AsS3 + 3H2O
Sb2S3 (antimonious sulphide) gives the same reaction with (NH4)2S as As2S3 but the following with KOH and NaOH,

Sb2S5 (antimonic sulphide) gives the following reactions with KOH:—
4Sb2S5 + 18KOH = 5K3SbS4 + 3KSbO3 + 9H2O
SnS (stannous bulphide) gives the following reaction with KOH:—
2SnS + 4KHO = K2SnO2 + K2SnS2 + 2H2O
SnS2 (stannic sulphide) with (NH4)2S and KOH.
SnS2 + (NH4)2S = (NH4)2SnS3
3SnS2 + 6KHO = 2K2SnS3 + K2SnO3 + 3H2O
NaOH reacts in a similar manner to KOH with these sulphides.
From these alkaline solutions the sulphides are thrown down unchanged by dilute HCl.
K3AsO3 + K3AsS3 + 6HCl = As2S3 + 6KCl + 3H2O
3KSbS2 + KSbO2 + 4HCl = 2Sb2S3 + 4KCl + 2H2O
5K3SbS4 + 3KSbO3 + 18HCl = 4Sb2S5 + 18KCl + 9H2O
K2SnO2 + K2SnS2 + 4HCl = 2SnS + 4KCl + 2H2O
2K2SnS3 + K2SnO3 + 6HCl = 3SnS2 + 6KCl + 3H2O
These sulphides are then treated with strong HCl; As2S3 is not acted upon, but the sulphides of Sn and Sb are decomposed.
Sb2S3 + 6HCl = 2SbCl3 + 3H2S→
Sb2S5 + 10HCl = 2SbCl5 + 5H2S→
SnS+ 2HCl = SnCl2 + H2S→
SnS2 + 4HCl = SnCl4 + 2H2S→
These chlorides are filtered off, diluted, and a piece of Zn foil and Pt foil are placed in contact in the solution, when the Sb is deposited on the Pt foil by galvanic action and the Sn on the Zn foil. The Sb is dissolved off the Pt with HNO3, and then precipated by H2S gas being passed through the solution, and Sn is dissolved in as little hot HCl as possible, and HgCl2 is added to this solution.
SnCl2 + 2HgCl2 = SnCl4 + 2HgCl
Division II. contains the sulphides of Hg, Pb, Bi, Cd and Cu, which are decomposed with HNO3, with the exception of HgS; this only upon prolonged boiling is partially converted into the nitrate. PbS may be partly oxidised into PbSO4.
PbS + 2NHO3 = Pb(NO3)2 + H2S→
Bi2S3 + 6HNO3 = 2Bi(NO3)3 + 3H2S→
CdS+ 2HNO3 = Cd(NO3)2 + H2S→
CuS + 2HNO3 = CU(NO3)2 + H2S→
HgS is dissolved in aqua regia, then diluted, and a strip of Cu placed in the solution.
HgCl2 + Cu = CuCl2 + Hg
Pb is precipitated by H2SO4, alcohol being added to completely precipitate it as PbSO4.
Pb(NO3)2 + H2SO4 = PbSO4 + 2HNO3
Bi is precipitated by NH4OH.
2Bi(NO3)3 + 6NH4OH = Bi2(HO)6 + 6NH4NO3
Bi2(HO)6 + 6HCl = 2BiCl3 + 6H2O
BiCl3 + H2O = BiOCl + 2HCl
Cd is precipitated from the ammoniacal solution by passing H2S gas through it.
Cd(NO3)2 + 2NH4OH = Cd(OH)2 + 2NH4NO3
Cd(OH)2 is soluble in the excess of NH4OH.
Cd(OH)2 + H2S = CdS + 2H2O
Cu gives a deep blue solution with excess of NH4OH.
Cu(NO3)2 + 4NH4OH = Cu(NO3)2, 4NH3, H2O + 3H2O
This blue solution is decolorised by the addition of KCN, when the Cu is converted into a double cyanide, having the formula 2KCN.Cu(CN)2, which passes quickly into the cuprous form, Cu2(CN)2.6KCN. CuS is not precipitated by H2S when there is an excess of KCu in solution, hence Cd can be separated. K4Fe(CN)6 precipitates cupric ferrocyanide. Cu2Fe(CN)6, insoluble in acids, but decomposed by alkalis, so the solution is made acid with CH3COOH before adding K4Fe(CN)6.
REACTIONS OF GROUP IIIa.
In this group the hydroxides of Al, Fe and Cr are dissolved in HCl.
Al2(HO)6 + 6HCl = Al2Cl6 + 6H2O
Cr2(HO)6 + 6HCl = Cr2Cl6 + 6H2O
Fe2(HO)6 + 6HCl = Fe2Cl6 + 6H2O
KOH and NaOH produce the same precipitate as NH4OH, only the hydroxides of Al and Cr are readily soluble in excess of these reagents.
Al2(OH)6 + 6NaOH = Al2O3.3Na2O + 6H2O
Cr2(OH)6 + 2KOH = Cr2O3.K2O + 4H2O
Cr2(HO)6 can be precipitated by boiling, while the sodium aluminate remains in solution.
The hydroxides of Cr and Fe are dried and then fused with Na2CO3 and KNO3.
Cr2O3 + 2Na2CO3 + O3 = 2Na2CrO4 + 2CO2
The Fe2O3 remains unchanged.
Na2CrO4 is dissolved out with water, and CH3COOH and Pb (C2H3O2)2 added to the solution.
Na2CrO4 + Pb(C2H3O2)2 = PbCrO4 + 2NaC2H3O2
Fe2O3 is dissolved in HCl, and K4Fe(CN)6 added to the solution.
2Fe2Cl6 + 3K4Fe(CN)6 = Fe4( Fe(CN)6)3 + 12KCl
Al2O3.3NaO is decomposed with HCl, and the Al is precipitated as hydroxide with NH4OH.
Al2O3 .3Na2O + 12HCl = Al2Cl6 + 6NaCl + 6H2O
Al2Cl6 + 6NH4OH = Al2(OH)6 + 6NH4Cl
REACTIONS OF GROUP IIIb.
In this group the sulphides of Zn, Mn, Ni and Co are decomposed with HCl, using a crystal or two of KClO3 when the latter two sulphides are present.
ZnS + 2HCl = ZnCl2 + H2S→
MnS + 2HCl = MnCl2 + H2S→
The hydroxides of Mn, Ni, and Co are precipitated by the addition ol NaOH or KOH; but the hydroxide of Zn is soluble in excess of either of these reagents.
Zn(OH)2 + 2NaOH = ZnNa2O2 + 2H2O
MnCl2 + 2NaOH = Mn(OH)2 + 2NaCl
NiCl2 + 2NaOH = Ni(OH)2 + 2NaCl
CoCl2 + 2NaOH = Co(OH)2 + 2NaCl
The solution containing the ZnNa2O2 is filtered, and the Zn is precipitated by passing H2S gas through it.
ZnNa2O2 + 2H2S = ZnS + Na2S + 2H2O
The hydrates of Mn, Ni and Co are then converted into chlorides by treating them with dilute HCl. The solution is nearly neutralised with NH4OH, and an excess of (NH4)C2H3O2 is added, which converts the MnCl2 into the acetate, from which solution MnS is not precipitated by passing H2S gas.
MnCl2 + 2(NH4)C2H3O2 = Mn(C2H3O2)2 + 2NH4Cl
The solution of Mn(C2H3O2)2 is then filtered off from sulphides of Ni and Co, and the Mn is precipitated as carbonate by the addition of Na2CO3.
Mn(C2H3O2)2 + Na2CO3 = MnCO3 + 2NaC2H3O2.
The NiS and CoS are converted into chlorides by treating with HCl and KClO3; Cl is boiled off, and the excess of acid is neutralised by the addition of Na2CO3. An excess of KCN solution is then added, forming double cyanides of Ni and Co.
NiCl2 + 4KCN = 2KCN.Ni(CN)2 + 2KCl
CoCl2 + 6KCN = 4KCN.Co(CN)2 + 2KCl
To the solution containing the double cyanides is added an excess of NaOH and then Br water, which precipitates the Ni as the hydrated sesquioxide and oxidises the potassium cobalto-cyanide into the potassium cobalti cyanide.
2K2Ni(CN)4 + 2NaOH + Br2 + 4H2O = Ni2(OH)6 + 4KCN + 2NaBr + 4HCN
2K4CO(CN)6 + 2NaOH + Br2 = 2K3Co(CN)6 + 2KOH + 2NaBr.
REACTIONS OF GROUP IIIc.
In this group the phosphates and hydrates are dissolved in HCl and the excess of acid is neutralised with Na2C03, then a solution of NaC2H3O2 and CH3COOH is added. Al, Cr, and Fe are precipitated as phosphates.
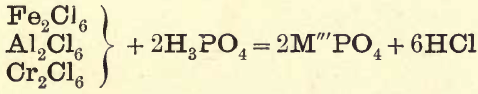
If there is excess of H3PO4 in solution, or none of the above bases are present, then Fe2Cl6 is added, drop by drop, as long as a precipitate falls; when a yellow colour appears, ferric acetate is being formed, in which ferric phosphate is soluble, therefore care must be taken only to add sufficient Fe2Cl6 to precipitate the H3PO4 in solution. All the phosphoric acid being removed, chlorides of Groups III. and IV. only remain, and are treated as in the General Table.
REACTIONS OF GROUP IV
In this group the carbonates of Ba, Sr and Ca are decomposed with HCl, and the solution is divided into two portions:—
1st portion—CaSO4 solution is added to test for presence of Ba.
BaCl2 + CaSO4 = BaSO4 + CaCl2
2nd portion.—NH4OH is added to neutralise excess of HCl, and then CH3COOH in excess is added to convert to acetates.
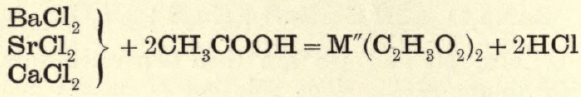
From the solution of acetates, K2Cr2O7 throws down BaCrO4.
2Ba(C2H8O2)2 + K2Cr2O7 + H2O = 2BaCrO4 + 2KC2H3O2 + 2CH3COOH.
Sr is then thrown down by the addition of K2SO4.
Sr(C2H3O2)2 + K2SO4 = SrSO4 + 2KC2H3O2.
To the remaining solution an excess of NH4OH is added and (NH4)2C2O4, when the Ca is precipitated as oxalate.
Ca(C2H8O2)2 + (NH4)2C2O4 = CaC2O4 + 2(NH4)C2H3O2
