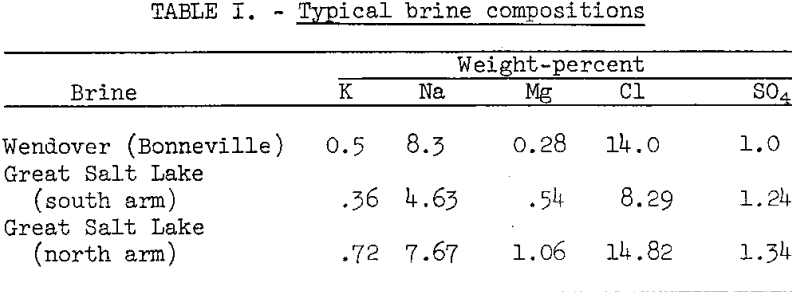
The U.S. Bureau of Mines and Great Salt Lake Minerals and Chemical Corp. developed a froth flotation process for concentrating potassium salts from Great Salt Lake solar evaporites containing about 5 percent K. The crude evaporites, which were predominately halite (NaCl) with smaller amounts of kainite (KCl·MgSO4·2·7H2O) and schoenite (K2SO4MgSO4·6H2O), were conditioned in a saturated brine solution, thus converting nonfloatable kainite to the floatable mineral schoenite. Schoenite was then selectively floated with a medium-molecular weight fatty acid. Continuous small-scale pilot plant testing indicated that 80 percent of the potassium could be recovered from the crude evaporites in flotation concentrates assaying about 11 percent K.
Overall Potash Flotation Program
The potash flotation program has been divided into three phases: (I) Laboratory and small-scale pilot plant tests (II) plant-size pilot tests, and (III) plant-scale operation. Because the flotation process will be the first of its kind in operation and comparable full-scale data are lacking, each phase requires technical and economic evaluation before proceeding to the next phase.
Bench-scale laboratory tests were performed to isolate and evaluate many of the variables associated with soluble salt flotation. These variables include collector type, type of evaporite harvested from the ponds, clay depression, grinding, froth control, flotation liquor, reagent conditioning, and conversion time.
The three types of brines evaluated as flotation liquors were (1) plant-end liquor (PEL), a saturated brine from the thickener overflow at GSL’s chemical plant, (2) conditioned PEL (PEL conditioned with the crude evaporite to be floated prior to flotation), and (3) recycled flotation liquor (recycled PEL). Table III lists the average compositions of the three flotation liquors used in the bench-scale experiments.
The standard test procedure was to pebble mill 500 grams of crude salt at 50 percent solids for 10 minutes, condition 10 minutes in a Fagergren flotation cell with the collector and other reagents, float a “rougher” concentrate for 5 minutes, recondition with the collector, and refloat a second rougher for 10 minutes. These conditions were varied to meet the need of a given set of test conditions, although milling for 10 minutes was sufficient to prepare 65-mesh material which would free the bulk of the potassium minerals from the halite.
Laboratory Results
Initial flotation tests were performed to determine a suitable collector for selective flotation of potassium from the crude evaporites. Neofat 8, a crude caprylic acid (an 8-carbon fatty acid), proved to be the most selective collector, particularly for schoenite mineral, which was determined by petrographic examination to be the most flotable potassium salt in the crude evaporites.
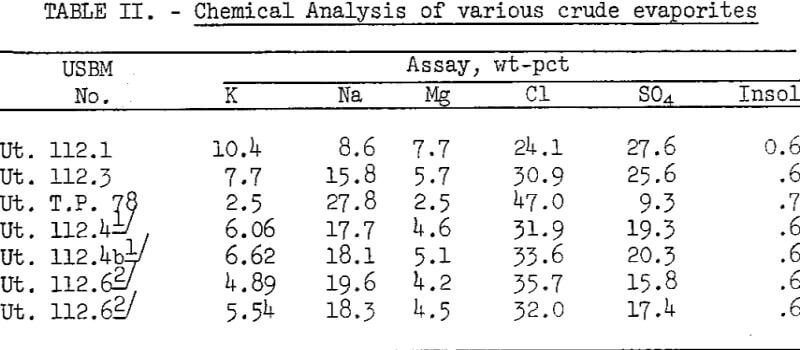
Flotation of crude salts Ut. 112.1 and Ut. 112.3, containing 10.4 and 7.7 percent K, respectively, produced concentrates having greater than 12 percent K but lower than 75 percent recovery. Crude salts of these potassium contents would not normally be used as flotation plant feed because they could be fed directly to the chemical plant producing K2SO4. The results do indicate that these higher grade salts can he floated by the proposed method if necessary.
The flotation liquors fresh PEL, conditioned PEL, and/or recycled flotation liquor (also referred to as recycled PEL caused differences in flotation product grades and recoveries and also influenced the conversion of potassium salts to schoenite. Temperature caused concentration changes in these brines and may well have some effect on kainite to schoenite conversion rates. Relative ion concentrations in these brines also depended on the crude salt being floated or on the crude salt with which they have come in contact prior to flotation.
Generally, the results indicated increases in K, Mg, and SO4 in the filtrates whenever decreases of these ions occurred in the calculated head. Decreases in Na and Cl ion concentrations in the filtrates generally accompany increases of these ions in the calculated heads. This was most apparent when fresh PEL was used as flotation liquor.
The results indicate that extended conditioning time of crude salt Ut. 112.4b containing 6.6 percent K and 18.1 percent Na increased total sodium recovery from 26 percent at 15 minutes total conditioning to 52 percent at 95 minutes conditioning. Potassium recovery increased from 85 to 90 percent for these respective conditioning times. Tests with crude salt Ut. 112.6 containing 5-6 percent K and 17.8 Na showed increased sodium recovery of 55 percent at 15 minutes conditioning time, compared with 28-percent recovery at 6 minutes time. Only slight changes in potassium recovery occurred, while potassium grade decreased at the shorter conditioning and flotation time.
Pilot Plant Tests
Upon completion of the bench-scale tests, a 100-pound-per-hour flotation pilot plant was assembled at the Bureau of Mines Research Center in Salt Lake City, Utah, to beneficiate evaporites from GSL’s 1973-74 pond harvest. The process included kainite to schoenite conversion in fresh PEL or recycled flotation liquor, followed by anionic schoenite flotation from crude halite salt. The generalized pilot plant flowsheet in figure 2 consists of (1) harvesting and crushing, (2) grinding, (3) conditioning, (4) flotation, and (5) brine recovery. The photograph in figure 3 shows the physical layout of the pilot plant at startup; however, the flotation cell scheme was changed to include cleaner and scavenger steps.
Because the crushed ore hardened in the storage bins, recrushing in a hammer mill (without grates) to produce 1-inch feed material was necessary. The crude salt feed rate was generally 90 pounds per hour; however, feed rates were lowered at one time to 60 pounds per hour. The crude salt was ground with 282 pounds of rods at 28.5 rpm at 50 to 60 percent solids to produce a 2-percent plus 48-mesh and 81-percent minus 150-mesh material. During this grinding step, the bulk of the kainite converts to schoenite.
The conditioned pulp (about 25 percent solids) was fed directly into No. 5 Denver Sub A flotation cells. These cells were used because of the flexibility in changing flowsheet arrangement. Their distinct disadvantage was their tendency to “salt up” during extended flotation periods.
Several flotation flow schemes were tested. The best potassium upgrading resulted when using a single cleaner cell, two rougher cells, and two or three scavenger cells. The cleaner concentrate was pumped to a drum vacuum filter, and the final tailing was pumped to a thickener which in this test work was a modified hydroseparator. The brines from vacuum filtration and the thickener overflow were pumped to a holding tank for recycling tests. Composite filter cakes and the thickener underflow were sampled every shift for assaying.
Starvation quantities of Neofat 8 collector (0.22 lb per ton) produced excellent concentrates of 15 percent potassium and 4.8 percent sodium at recoveries of 64 and 7.6 percent, respectively. Collector additions of 0.37 and 0.57 pound per ton produced concentrates containing 11.3 to 12.7 percent potassium and 9.2 to 9.9 percent sodium at the higher recoveries of 74 percent potassium and 10 to 12 percent sodium. The use of recycled brine which contains residual collector produced even lower quality potassium concentrates at a slight increase in recovery. Factors other than residual collector influence flotation and are discussed in the section on flotation liquors.