Table of Contents
The failure, or disintegration, of concrete in structures, even when the cement, sand, and coarse aggregate used have passed satisfactorily all tests and inspections, is not uncommon. Such failures occur even when proper methods of mixing and placing have been used and where weather conditions were correct. In these cases, the origin of the trouble must be sought in the cement, therefore some additional mode of determining the quality of cement must be employed.
The problem is to find a simple, usable; easily applied method that will supplement the standard tests. The solution of this problem involves a knowledge of the chemical composition of Portland cements, also of their constitution (or componential composition), as the behavior of a cement, when gaged, is dependent on the physical and chemical characters of the compounds of which it is composed, the proportions existing between them, and the fineness of grinding. It is likewise essential to know the character, behavior, and composition of the hydration, products of Portland cement.
The most promising method of attack makes use of chemical, mechanical, and petrographic methods. Chemical, by determining quantitatively not only the usual constituents, but the carbon dioxide and water. Mechanical, by determining the fineness with the air analyzer, which separates into fractions of various grain dimensions all of that portion which in the ordinary sieve test passes the 200-mesh sieve and constitutes at least 75 per cent, of the total cement. These fractions may be further studied chemically and petrographically and micrometric measurements made of the grains. Petrographic, by the method of immersion, and by making thin sections from test pats of both neat cements and standard-mortars.
Normal Portland cement is composed of mechanical mixtures of definite chemical compounds having constant chemical and physical properties. In the order of their cementing qualities these are Tri-calcium silicate, 3Ca0.SiO2, tricalcium aluminate, 3CaO.Al2O3, trical ferrite, 3CaO.Fe2O3, and calcium orthosilicate, beta form, 2CaO.Si In addition, there is a certain quantity (generally about 3 per cent.) gypsum. The magnesia, MgO, is in a state of solid solution the other components and is apparently without much effect in the quantities found in cements. Both potash and soda exist to a small degree detect flakes of quartz from the ball mills, metallic iron from the mac u particles of semi-fused coal ash from the fuel, etc. are sometimes found.
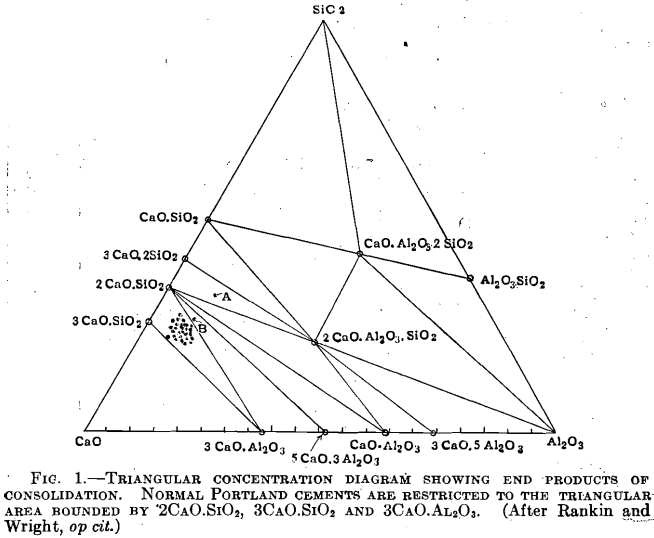
A long series of studies on the ternary system, lime-silica-alumina, and the system alumina-silica-magnesia, as well as studies of binary systems of the same components, by physicists, physical-chemists and chemists of the Geophysical Laboratory, the Bureau of Standards, and different universities, and a comparison study of Portland cements has shown quite definitely that the three constituents that form the bulk of normal Portland cement—lime, CaO, silica, SiO2, and alumina, Al2O3—exist as fixed components with definite chemical composition and constant optical properties.
A study of the analyses of standard brands of normal Portland cement will show that their percentages of lime, silica, and alumina amount to over 90 per cent. Perfectly sound Portland cement has been made of these three constituents alone; hence if the percentages of these constituents are recalculated to a basis of 100 per cent., neglecting the ferric and other oxides that are always present in small amounts, the resulting percentages may be plotted on the ternary concentration diagram.
Points thus plotted will fall within a restricted triangular area within the diagram, with three compounds forming the apices of the small triangle; viz., tricalcium silicate, 3CaO.SiO2, tricalcium aluminate, 3CaO. Al2O3, and dicalcium silicate (or calcium orthosilicate), 2CaO.SiO2, beta modification. Differences in the percentages of lime, silica, and alumina, within certain limits, do not cause the appearance of a fourth and new compound; they merely change the proportion between the three compounds mentioned; this is an important point. If, however, the difference in percentage composition is great enough, on recalculating the percentage of the three constituents (SiO2, Al2O3, CaO) to a 100- per-cent. basis and plotting the result, the point will fall outside of the area mentioned and different compounds may be expected to occur, some of which may be non-hydraulic.
The results of about fifty analyses made in the laboratory of the Board of Water Supply of the City of New York have been recalculated and plotted on the triangular concentration diagram in the manner described (some of the dots cover a number of plotted points); all the points so plotted fall within the triangular area mentioned except two, A and B, Fig. 1. One of these A is a cement with abnormally high silica (33.01 per cent.) and low lime (52.73 per cent.); the other B falls within the units of the composition usually set for Portland cement, but it also is a high in silica and too low in lime.
Hydration Products of Portland Cement
When mixed with water Portland cement undergoes the following changes:
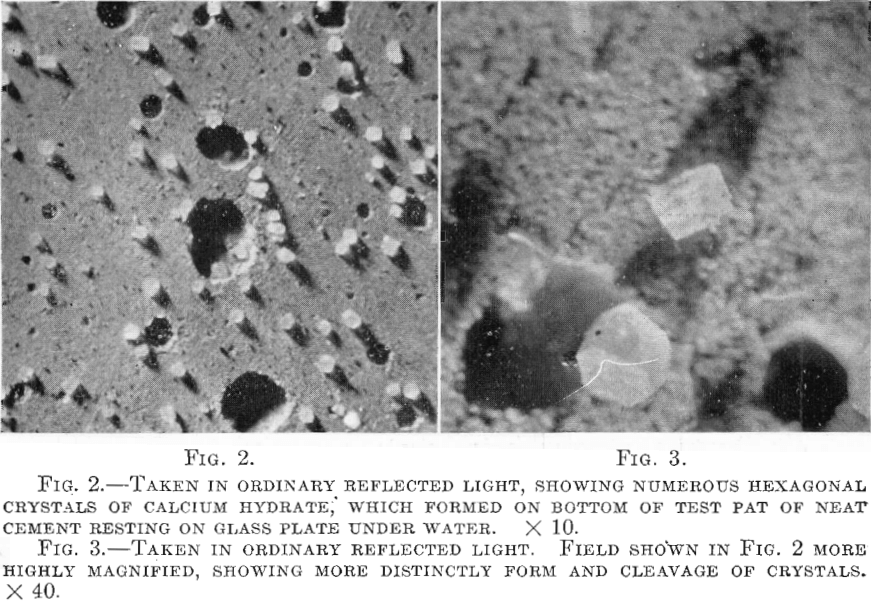
1. The tricalcium silicate, 3CaO.SiO2, loses a molecule of lime, which at the same time hydrates; the remainder of the compound unites with the added water to form an amorphous, indefinite, hydrated silicate of calcium which slowly loses more calcium hydrate by hydrolysis. The calcium hydrate thus formed may be partly crystalline and partly amorphous, or even almost wholly crystalline, dependent on the amount of water used and subsequent conditions of wetness. When partly or wholly crystalline, it is distributed through the concrete, and especially between the sand and aggregate and the cement matrix both in extremely fine and in relatively coarse and large crystals.
2. Tricalcium aluminate, 3CaO.Al2O3, when treated with water generates so much heat during its hydration as to expel some of the water as steam; at the same time, so rapid is the rate of hydration that an amorphous envelope of hydrated material coats each particle and tends to prevent further and complete hydration. High alumina cements generally set rapidly, and are “hot” cements. As gypsum seems to retard the rate of hydration of this component, it is added to all Portland cement clinker before grinding. The hydrated product is largely amorphous, but may be in small part crystalline; the amorphous product delivers calcium hydrate by hydrolysis.
3. Tricalcium ferrite, 3CaO.Fe2O3(?), hydrates to an indefinite, brown, lime-iron compound, quite amorphous, which delivers calcium hydrate by hydrolysis. The amount of calcium ferrite in ordinary Portland cements is never very large.
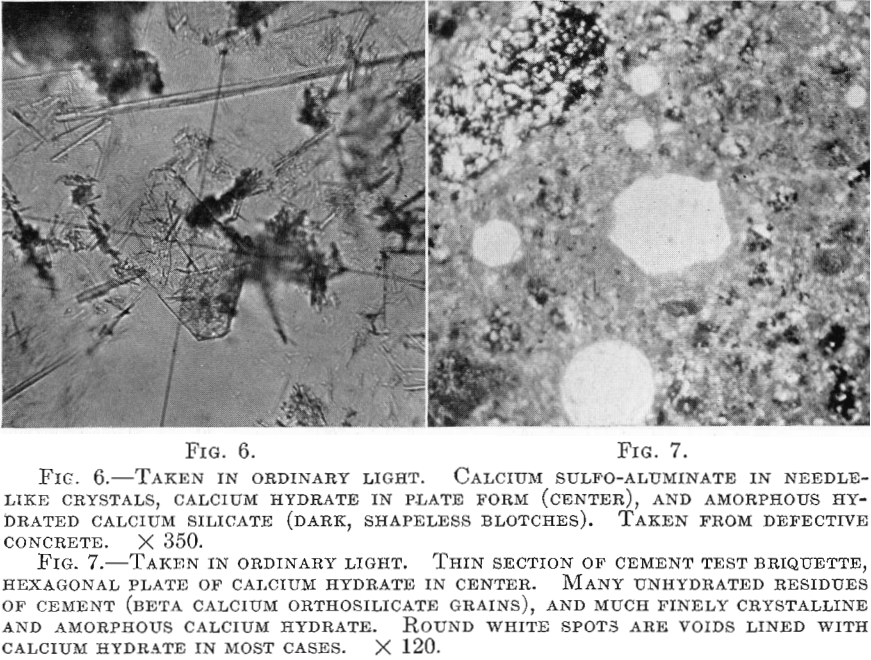
4. Beta calcium orthosilicate, 2CaO.SiO2, is the most difficultly hydratable component of cement. All of the unhydrated residues of cement clinker commonly seen (microscopically) in cement test pieces and in concrete consist of unhydrated granular aggregates of this component, which frequently encloses grains of the other components in such a way as to prevent them also from hydrating.
If the cement is ground sufficiently fine, beta calcium orthosilicate slowly takes up water of hydration and is converted to an amorphous-indefinite hydrated calcium silicate that, by hydrolysis, yields more or less calcium hydroxide. Seldom is the action complete, and in the case of rather coarse cements innumerable particles are wholly unhydrated, thus decreasing the efficiency of the cement.
5. Gypsum, CaSO4.2H2O, appears to control the rate of hydration of the tricalcium aluminate and the evolution of heat. Moreover, a chemical reaction between the gypsum and the hydrated aluminate is responsible for the development of silky needles of calcium sulfo-aluminate (3CaO. Al2O3.3CaSO4 + much H2O), a normal hydration product. Under some conditions this may become abnormal in size of crystals, quantity, and distribution, and one of the possible causes of disintegration of concrete
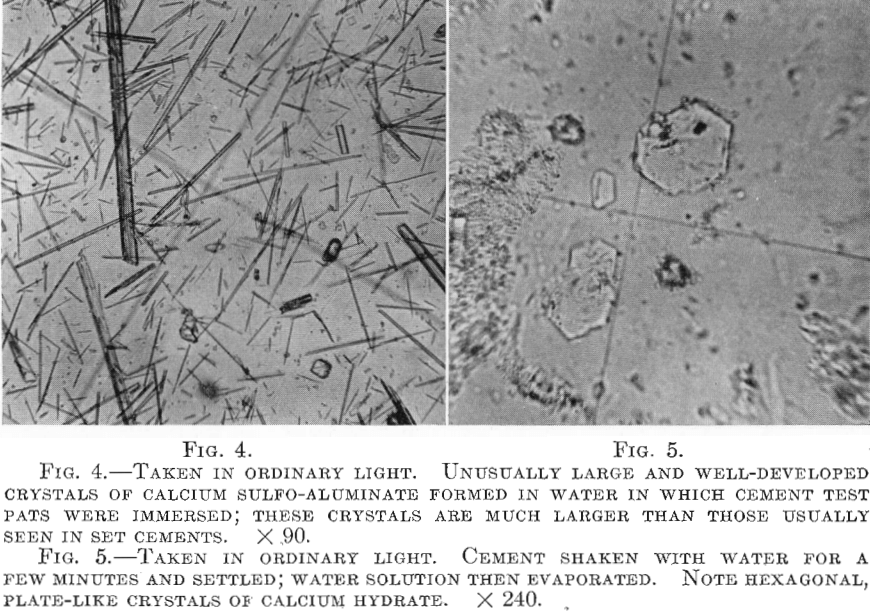
in certain situations. Some of these features are illustrated by Figs. 2 to 7, which show the character of the hydration products mentioned.
Recasting Cement Analyses
As the components of Portland cement have a definite composition, the various oxides found by chemical analysis must be uniformly distributed and united with one another according to fixed laws of chemical combination. Hence it should be possible to recast an analysis in much the same manner as rock analyses, and to obtain the percentage composition of the cement in terms of the actual compounds, instead of the oxides of the elements forming the mass.. This is accomplished by dividing the percentages of the various oxides by their molecular weights, obtaining thus their molecular numbers, or ratios, and then allotting the proper quantities of silica, alumina, lime, etc. to each in accordance with the various compounds that should form under the conditions of burning. Thus, sufficient lime is allotted to satisfy the requirements of CO2, Fe2O3, SO3, and Al2O3; the remaining lime is allotted to the silica to form both tricalcium silicate and calcium orthosilicate. This should be preceded, however, by a petrographic study of the cement in order to determine whether or not any abnormal components are present. For example, a cement analyzes as follows:
SiO2, 23.40 per cent.; Fe2O3, 3.46 per cent.; Al2O3, 5.54 per cent.; CaO, 60.79 per cent.; MgO, 2.46 per cent.; H2O, 2.59 per cent.; SO3, 1.71
per cent.; CO2, 0.45 per cent. A “recast” of the analysis will then be as follows:
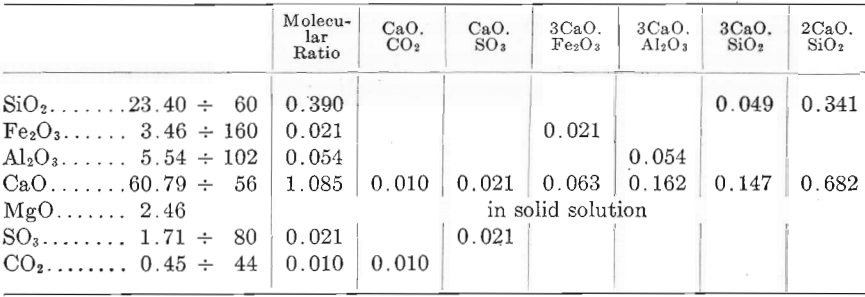
The percentage composition, in terms of the components, may be found by multiplying the molecular numbers allotted (or component constants) by the molecular weights of the compounds; thus, the molecular weight of tricalcium silicate, 3CaO.SiO2, is 228, which, multiplied
by the component constant (0.049) gives as a product 11.172, or 11.2 per cent. The componental composition of the cement is thus found to be: Tricalcium silicate, 11.2 per cent.; calcium orthosilicate, 58.7 per cent.; tricalcium aluminate, 14.6 per cent.; tricalcium ferrite, 6.9 per cent.; gypsum, 3.6 per cent.; calcium carbonate, 1.0 per cent.; magnesia, MgO, 2.46 per cent.
It has been established by experiment that the component of greatest cementing value is tricalcium silicate, and the component most difficultly hydratable is calcium orthosilicate. It follows, therefore, that the cementing value of any mixture of these components, such as Portland cement, is dependent on the proportions existing between the components, and the fineness of grinding of the mixture. In the example given, as the cement has only 11. 2 per cent, of tricalcium silicate (the best hydraulic, component) and 58.7 per cent, of calcium orthosilicate (the most difficultly hydratable component), particularly if relatively coarse, it should be regarded with suspicion.
Importance of Fine Grinding
The fineness of grinding is an important feature and one not properly determined by the standard sieve test; although three-quarters of the cement may pass the 200-mesh sieve, the bulk of it may be, and frequently is, too coarse for efficient work, especially if it is high in orthosilicate and low in tricalcium, silicate. When a relatively coarse cement of this character is used in concrete work, a petrographic study of thin sections cut from the concrete will prove the presence of a large amount of unhydrated cement clinker. In many cases from 25 to 40 per cent, of the cement is unhydrated or partly hydrated, resulting in a weak, permeable concrete mass that readily lends itself to attack by the agents of disintegration.
Limiting Ratios of Cement
By considering cements from the standpoint of their componental composition and selecting those with proper adjustments, between these components, it is possible to guard against subsequent disintegration of completed structures to a very considerable extent. As an aid to proper selection, a limiting ratio based on a minimum tricalcium silicate content may be used. If the percentages of lime, silica, alumina, and ferric oxide, the sum of which in all normal Portland cements always exceeds 90 per cent., are converted to molecular ratios, the quotient obtained by dividing the molecular ratio of the lime by the sum of the molecular ratios of the alumina, silica, and ferric oxide, may be used as a selective ratio. The writer suggests 2.4 as the limiting ratio. All cements having a lower ratio than this should be regarded with suspicion.
The use of the limiting ratio in this manner provides a simple, easy method of judging the quality of Portland cement, when used in connection with the usual standard tests; especially if the fineness of grinding is determined with the air analyzer. A more elaborate and careful study of the quality may be pursued by using petrographic methods in addition.
Results of Limiting Ratio Tests
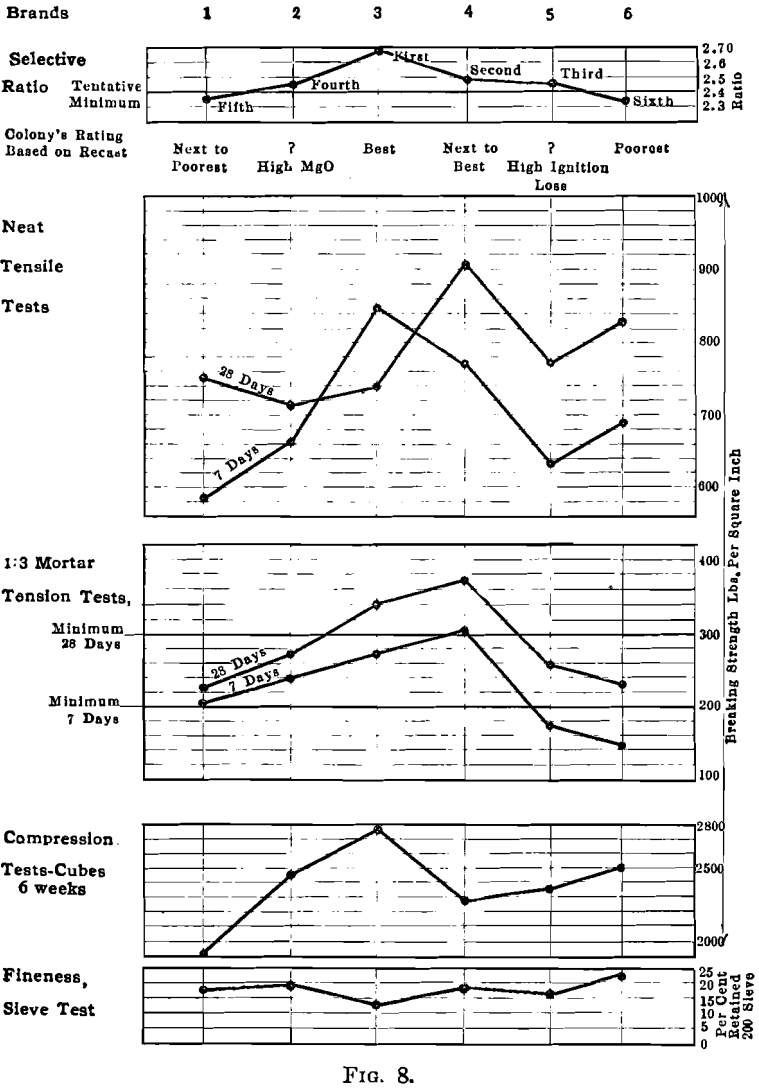
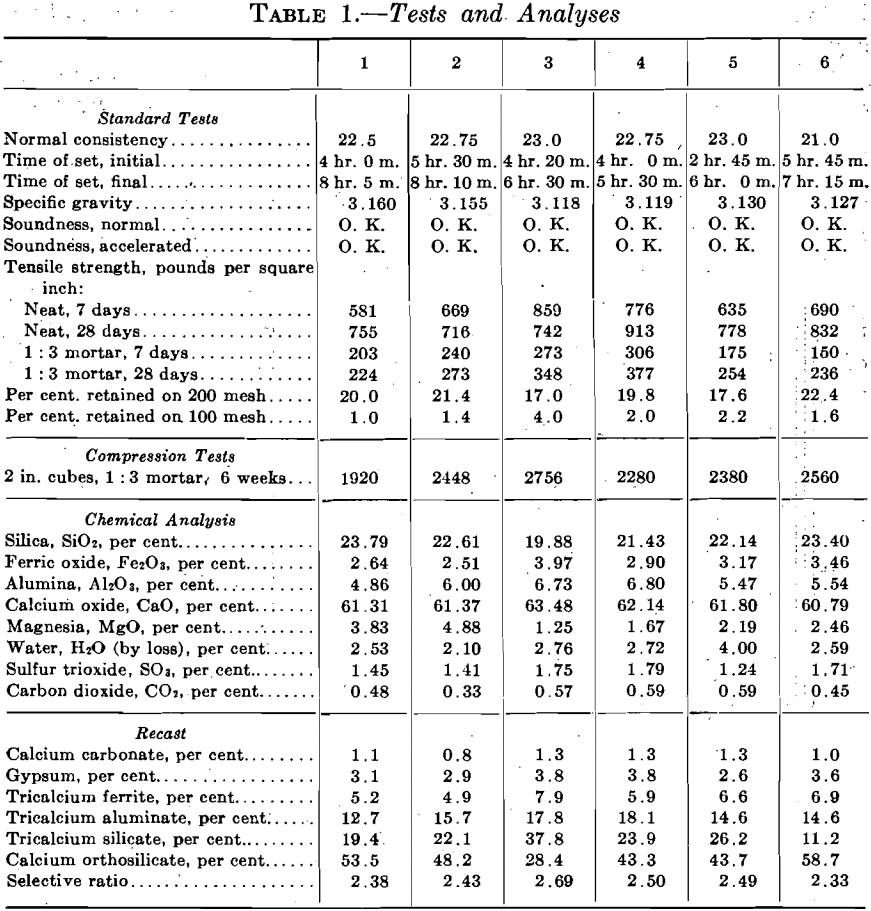
As an example of the practical application of this method there are included in Table 1 and Fig. 8 tests and analyses of six standard brands of Portland cement. These were rated by the writer solely from the interpretation of the chemical analyses; the cements themselves were not seen, no micrometric measurements for fineness were made, and the results of the tests were unknown at the time of rating. On the basis of the recasts and the ratios cements Nos. 3 and 4 were judged to be the best; Nos. 2 and 5 the next best; and Nos. 1 and 6 the poorest. The standard tests shown were made subsequently by the Testing Laboratory, Columbia University. The table and diagrams were compiled by the engineers for whom the ratings were made, and are used here by their permission.
To rate a cement solely from the chemical analysis, without having seen the cement, and without an opportunity for petrographic study is a severe handicap. Under the conditions, therefore, it is judged that the conclusions as to the quality of these six cements check very closely the results obtained by standard tests. The method contemplates the use of the air analyzer, microscopic inspection, and the interpretation of the chemical analyses; results obtained are so suggestive that further work is now in progress.
