Table of Contents
An exploration program initiated by Johns-Manville Corp. resulted in the discovery of significant occurrences of platinum-group elements in the Stillwater complex of south-central Montana. The mineralization occurs in a horizon of relatively sulfide-rich anorthositic rocks within the Banded Zone of the complex. The favorable horizon is continuous over a strike length of 24 miles. The horizon exposed in the Johns-Manville West Fork exploration adit has an estimated grade of 0.47 oz Pt-Pd/ton and a Pd-to-Pt ratio of 3.5 across a width of 6 feet and along a strike length of 2,100 feet.
Production of palladium and platinum from the Stillwater complex is of great national interest because it could supply most of the U.S. requirements for palladium and about one-fourth of the requirements for platinum. Without this palladium-platinum supply, the United States will continue to import most of its primary platinum-group metals in the future. The nature and distribution of platinum-group metal deposits in the world indicate that South Africa will remain the prime supplier of platinum and the USSR the prime supplier of palladium.
The objective of this investigation was to devise a method for preparing Pt-Pd concentrate from the Stillwater complex ore.
General Geology and Mineralogy
The Stillwater igneous complex is an intrusion of lopolithic form. Differential segregation and crystal settling of different magmatic minerals resulted in a sequence of sedimentary-like layers or bands totaling about 25,000 feet in exposed thickness. The minerals containing the platinum-group metal values are concentrated in several of the bands. The estimated composition of the Stillwater complex magma is very similar to that of the Bushveld complex of South Africa. Likewise, with respect to layering, the Stillwater complex and the Bushveld complex closely parallel each other.
A platinum-palladium bearing sulfide horizon occurring within a lower anorthosite member of the Banded Zone is being explored by Johns-Manville Corp. This report represents the results of mineral beneficiation tests on a sample taken by Johns-Manville from its West Fork exploration adit driven along the sulfide horizon. Analysis of the sample showed Pt. 0.08 oz/ton; Pd, 0.35 oz/ton; Au, 0.02 oz/ton; Cu, 0.06 wt-pct; and Ni, 0.11 wt-pct. For comparison, representative ore from the Merensky Reef in the Bushveld complex of South Africa is reported to contain Pt. 0.13 oz/ton; Pd, 0,05 ox/ton; Au, 0. 01 oz/ton; Cu, 0.11 wt-pct; and Ni, 0.19 wt-pct.
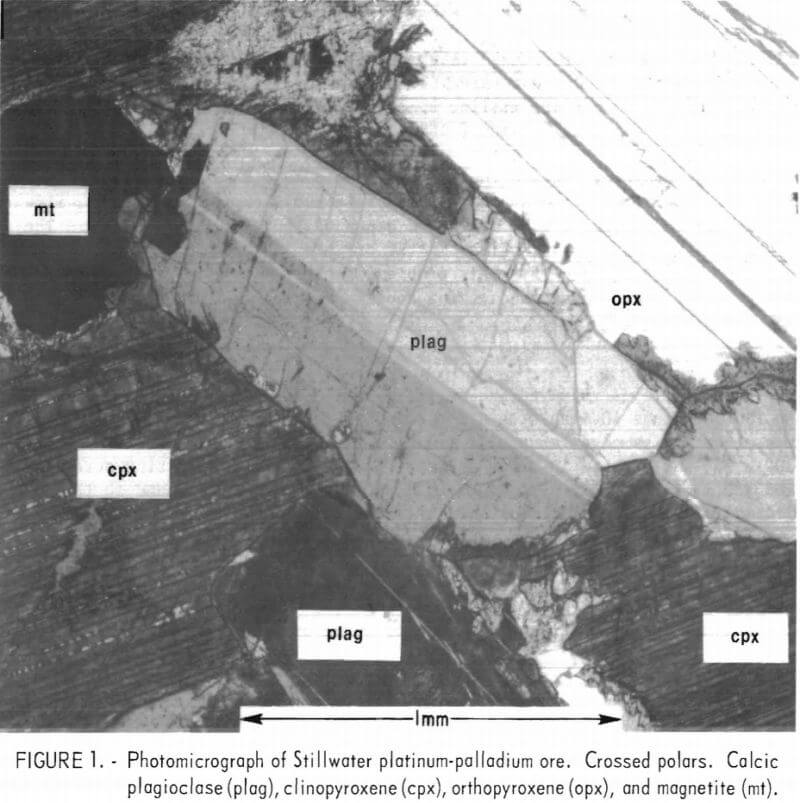
Mineralogical examination of a sample of the Stillwater ore showed that the major minerals are calcic plagioclase, clinopyroxene, orthopyroxene, and olivine. A minor amount of serpentine is also present. Accessory minerals include pyrrhotite, pentlandite , pyrite, chalcopyrite, and magnetite. The sulfides make up about 1 wt-pct of the rock. Figure 1 shows the distribution of major minerals and their textural relations. Because of the mineral composition and texture, the rock is classified as a gabbro. Specific gravity of the sample was 2.8.
Using a combination of hydroclassification, heavy-liquid separation, and magnetic separation, mineral samples were prepared to determine the mode of occurrence of the platinum-group metals and to obtain information for the formulation of a minerals beneficiation scheme. Electron probe microanalysis of fractions from the heavy-liquid separation of a minus 100- plus 200-mesh (Tyler standard screen) sample showed that most of the sulfide minerals (pentlandite, pyrrhotite, pyrite, and chalcopyrite) were contained in mixed grains with a silicate matrix. Examination of one sample showed that most of the palladium was associated with the pentlandite whose composition, in wt-pct, was as follows: Ni, 34, Fe, 32, S, 31, Pd, 1.3, and Co, 0.7. Also, two grains of labradorite, each containing 1 grain of a platinum telluride mineral, were observed. Likewise, 1 grain of a Pt, Pd, Ni sulfide mineral was found locked in a grain of Ca, Mg, Al silicate mineral. These occurrences were typical of platinum-group element mineralization in the Banded Zone. Cabri and LaFlamme have identified braggite [(Pd,Pt)S] and the tellurides moncheite (PtTe2), kotulskite (PdTe), and merenskyite (PdTe2) in rock specimens from the Banded and Upper Zones of the Stillwater complex.
The sulfide minerals were disseminated throughout the rock with a few occurrences of coarse-grained sulfides. Microscopic examination of screened fractions of crushed ore showed that most of the sulfides could be liberated at 200-mesh. The Bond Index value for the ore ground to minus 200-mesh was 16 kwhr/ton. Most ore milling operations have work indices ranging from 10 to 12, with extremes between 8 and 22.
Because most of the platinum-group bearing sulfides occurred as fine grains and required grinding to approximately 200-mesh for liberation, flotation was investigated as a means for concentrating the metal values. The low concentration of metal values in the ore made it difficult to obtain consistent analytical results. This problem was resolved in most cases by carefully mixing the products and taking duplicate samples.
Flotation Test Equipment & Procedure
The sample as received was approximately minus ¾-inch and was crushed to 100 pct minus 10-mesh with a cone crusher and rolls. The minus 10-mesh ore was mixed and sampled, and split in 30-lb (13.6-kg) lots as needed. Each 30-lb lot was crushed in rolls and screened on a Sweco vibrating screen to 100 pct minus 65-mesh. The minus 65-mesh product was dry ground to the desired sieve size in a steel ball mill operating in closed circuit with a Sweco vibrating screen. The ground ore was mixed on a large rolling cloth, and the charges to be used for flotation tests were split out, weighed, and bagged. Dry grinding was chosen for the final comminution step because of greater convenience than wet grinding in sizing, sampling, and handling of the product. In the course of the study, some flotation tests were made on wet-ground ore. Metallurgical results were similar to those obtained using dry-ground ore.
Both conditioning and rougher flotation were conducted in Denver No. 12 laboratory flotation cells having stainless steel impellers, stators, and tanks. Tests were made using 1,600- and 5,000-gram charges or ore. The 1,600-gram tests were made in a 5.5-liter tank fitted with a 2-7/8-inch- diameter impeller and a 3-1/8-inch-diameter stator. The 5,000-gram tests were made in a 10-liter tank fitted with a 3¾-inch-diameter impeller and a 4¼-inch-diameter stator. Impeller speed was 2,100 rpm in the 1,600-gram tests and 1,800 rpm in the 5,000-gram tests. To prepare cleaner concentrates, rougher concentrates from both 1,600- and 5,000-gram tests were conditioned and floated in a 2-liter stainless steel tank having an impeller mechanism operating at 1,800 rpm.
Pulp pH in all flotation tests was determined with a phenaphtazine indicator paper. The pH’s were checked with a Corning Digital 109 general-purpose pH meter.
Preliminary Flotation Tests
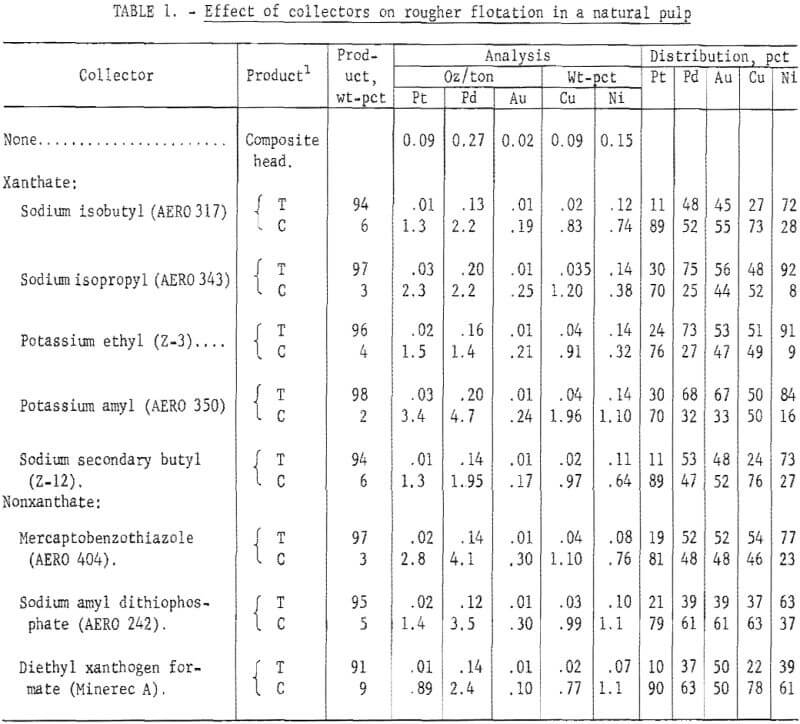
Preliminary flotation tests using batches of 1,600 grams of ore that were dry ground in stages to minus 200-mesh (67 pct minus 325-mesh) were made to screen collectors. The following experimental conditions were used: solids content, 40 pct; amount of collector, 0.1 lb/ton of ore; and polypropylene glycol methyl ether frother (Dowfroth 250), 0.015 lb/ton of ore. Both conditioning and flotation time were 10 minutes and at the natural pulp pH of 6. The xanthate collectors tested were sodium isobutyl (AERO 317), sodium isopropyl (AERO 343), potassium ethyl (Z-3) , potassium amyl (AERO 350), and sodium secondary butyl (Z-12). The nonxanthate collectors tested were mercaptobenzothiazole (MBT) (AERO 404), sodium amyl dithiophosphate (AERO 242), and diethyl xanthogen formate (Minerec A). Results of the preliminary tests are shown in table 1. Distribution of palladium values in the concentrates ranged from 26 to 63 pct and that of platinum from 72 to 90 pct. Best platinum and palladium recoveries were obtained from diethyl xanthogen formate as the collector. Increasing the amount of each collector to 0-2 lb/ton of ore did not yield significantly lower grade Pt-Pd tailings.
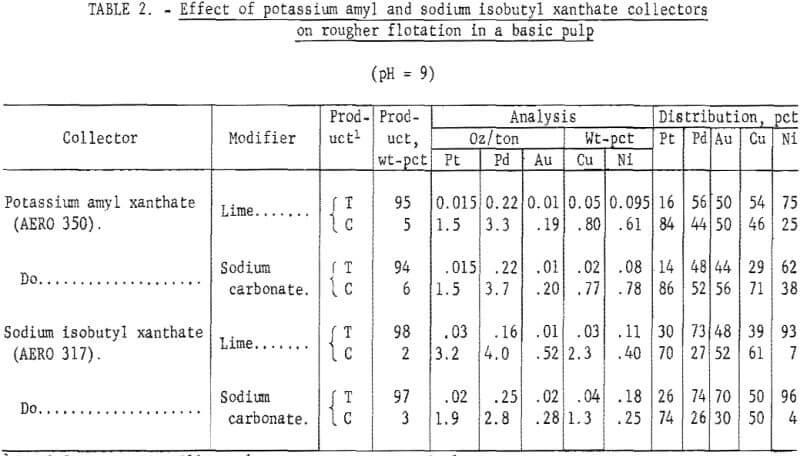
Tests were made with potassium amyl xanthate and sodium isobutyl xanthate collectors at a pH of 9. Lime (2.5 lb/ton of ore) was added to increase the pH. Other test conditions were the same as those used in the natural pH tests.
Because lime has been reported to act as a depressant in the flotation of iron sulfide minerals and a high sulfide recovery in the concentrate is desired for the recovery of platinum-palladium values, tests employing sodium carbonate were made at a pH of 9. Results are given in table 2. Palladium recoveries were low with both collectors and ranged from 16 to 51 pct. With potassium amyl xanthate, no significant difference in concentrate grade and platinum-palladium recovery was obtained by using either sodium carbonate or lime. With sodium isobutyl xanthate, a higher grade platinum-palladium concentrate was obtained with lime than with sodium carbonate. In some exploratory tests, addition of copper sulfate activator to either natural or basic pulps did not improve flotation.
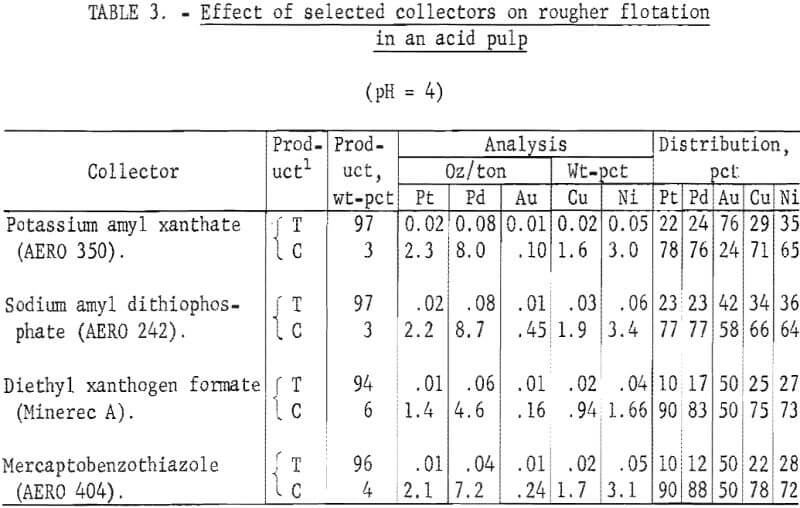
As described previously, most of the platinum and palladium values in the Stillwater ore are associated with the pentlandite and pyrrhotite minerals. Studies by Chang have shown that acid addition greatly increases the floatability of pyrrhotite and that flotation of pyrrhotite with potassium amyl xanthate as a collector has a critical pH value of 4.5. Tests using potassium amyl xanthate, sodium amyl dithiophosphate, diethyl xanthogen formate, and MBT collectors with polypropylene glycol methyl ether frother were made at a pulp pH of 4. Grind, solids content, amount of collector and frother added, and flotation time were the same as those used in previous tests. The pH of the pulp was adjusted to 4 by the addition of 22 lb H2SO4 per ton. Acid and collector were added simultaneously, and conditioning time was 10 minutes. Results are given in table 3. Significantly higher recoveries of platinum and palladium values were obtained in the concentrate. The increase in palladium content of the concentrate and the markedly higher palladium recovery were of particular significance. MBT and diethyl xanthogen formate collectors yielded significantly higher platinum and palladium recoveries than those obtained with the other collectors. Flotation with MBT collector resulted in a higher grade concentrate than that obtained with diethyl xanthogen formate.
In other tests, a modified procedure was used in which the pulp was conditioned with 22 lb H2SO4 per ton of ore, the solids settled, and the supernatant decanted. The solids were repulped with fresh water and then subjected to flotation. The procedure minimizes contact of acid with equipment in the flotation circuit. Either MBT or diethyl xanthogen formate and polypropylene glycol methyl ether frother were used in these tests. Tailings obtained with the modified procedure were higher in platinum and palladium content (0.02 oz/ ton Pt and 0.11 oz/ton Pd) than those obtained from flotation in high acid pulps.
Substituting sulfurous acid for sulfuric acid to decrease the pH to 4 and floating with a suite employing MBT was not successful. Recoveries of platinum and palladium were 77 and 46 pct, respectively.
Optimization of Rougher Flotation
with Mercaptobenzothiazole Collector and Sulfuric Acid Modifier
Preliminary tests showed that the combination of MBT collector, sulfuric acid, and polypropylene glycol methyl ether frother was most effective in concentrating Pt-Pd minerals. The use of xanthogen formate as collector gave recoveries comparable to those obtained with MBT, but the grade of concentrate was lower. Tests, therefore, were made to determine the best addition level of MBT and sulfuric acid as well as the best grind and pulp solids in the rougher flotation circuit.
Six series of tests were made to investigate the following parameters; (1) conditioning time with MBT and sulfuric acid added simultaneously to the pulp, (2) flotation time, (3) amount of MBT required, (4) amount of sulfuric acid required, (5) pulp solids content, and (6) fineness of grind. For all tests, except for fineness of grind, 1,600-gram charges were stage dry ground to minus 200-mesh. In the fineness of grind tests, the charges were stage dry ground to minus 100, 150, 200, 270, and 325 mesh. A solids content of 40 pct was used in series 1 through 4 and in series 6. In series 1, investigation of conditioning time, the following conditions were used; MBT, 0.125 lb/ton of ore; sulfuric acid, 22 lb/ton of ore; polypropylene gylcol methyl ether frother, 0.015 lb/ton of ore; and flotation time, 10 minutes. In each subsequent series (2-6), the best conditions as determined in the preceding series were used. Results are shown in figures 2-7, Best conditions determined from all tests are summarized as follows: conditioning time, 10 minutes; flotation time, 10 minutes; amount of MBT, 0.125 lb/ton of ore; amount of sulfuric acid, 22 lb/ton of ore; solids concentration, 40 pct; and grind, minus 200-mesh (67 pct minus 325-mesh). The amount of polypropylene glycol methyl
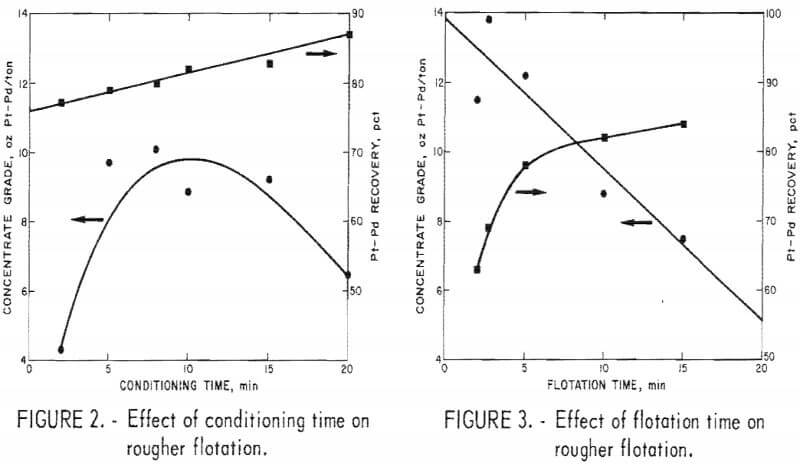
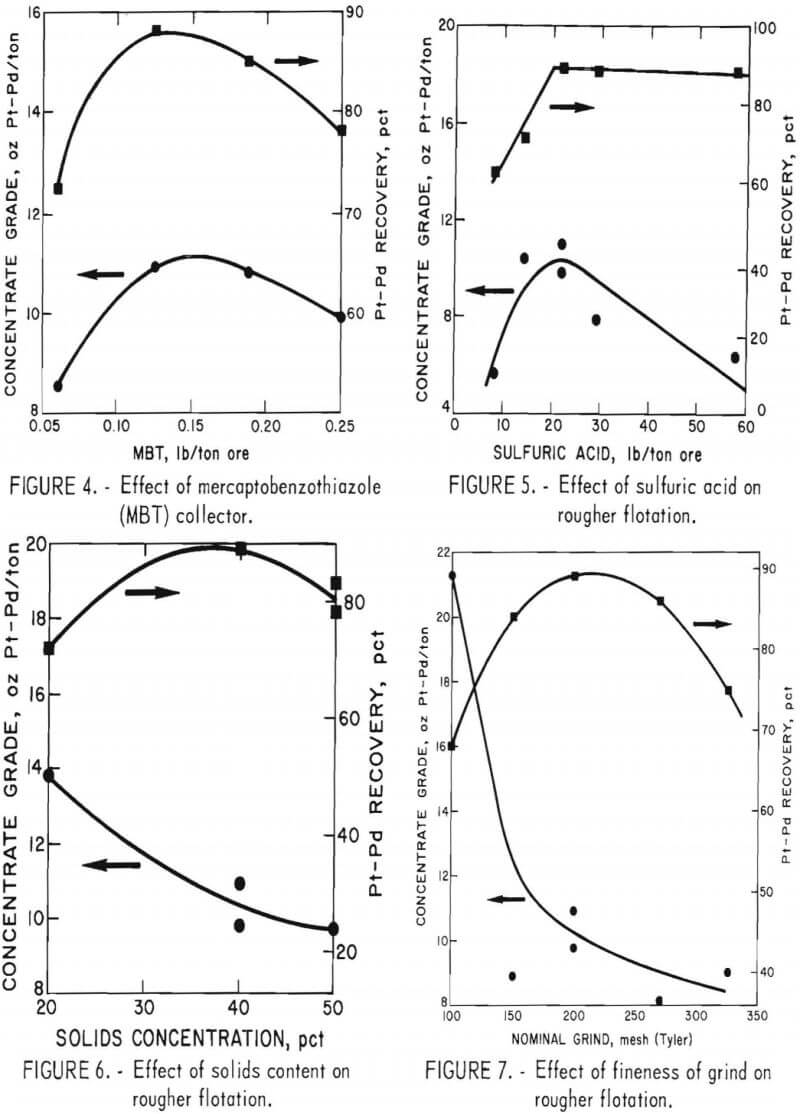
ether frother used was 0.015 lb/ton of ore. This quantity gave acceptable results and was not investigated as a variable. Although the concentrate grade was higher with a shorter flotation time, recovery of Pt-Pd values was considerably lower. Likewise, an increase in concentrate grade was obtained by floating at 20 pct rather than 40 pct solids, but Pt-Pd recovery decreased.
Effect of Recycling Water in Rougher Circuit on Flotation
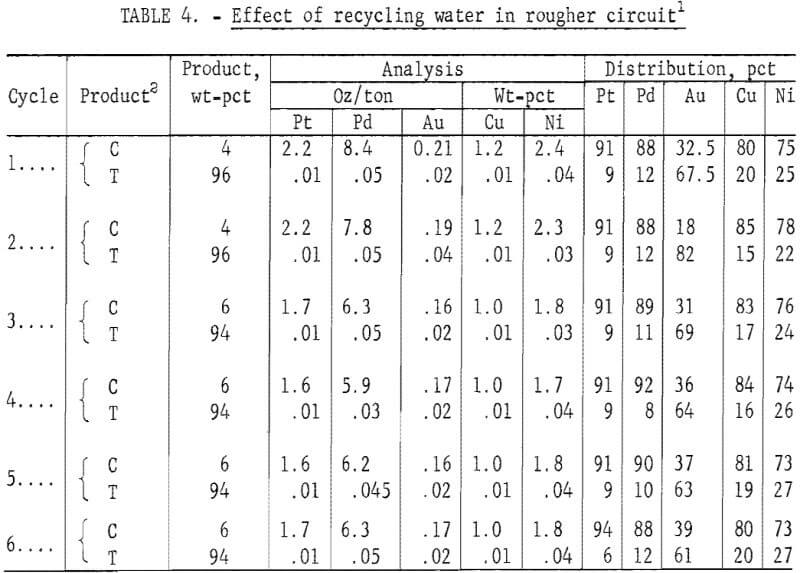
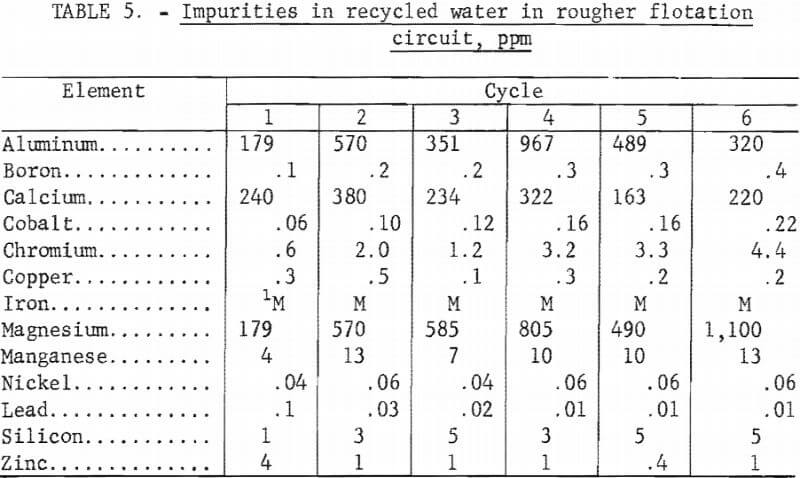
Using the best conditions determined earlier, a series of six flotation tests was made to determine the effect on concentrate grade and Pt-Pd recovery of recycling water in the rougher circuit. In each test, a pulp pH of 4 was maintained by adding 22 lb of sulfuric acid per ton of ore to the recycled water. The required amount of MBT collector was also added. Polypropylene glycol methyl ether added in the first two tests provided adequate frother for all subsequent tests. Results are shown in table 4. Although concentrate grades were slightly higher in cycles 1 and 2 than in the succeeding cycles, no deleterious effect on flotation was noted by recycling water in the flotation circuit. A sample of water from each test in the series was evaporated to dryness and analyzed for impurities by an optical emission spectrographic procedure. Results are shown in table 5. Except for magnesium and aluminum, no significant buildup of impurities was noted during the six test cycles.
Larger Scale Rougher Flotation Tests
In larger scale tests using the best conditions previously determined for the MBT-sulfuric acid flotation scheme, ore charges to the rougher flotation circuit were increased to 5,000 grams. The average results of 10 tests are given in table 6. Platinum-palladium recovery values were similar, but concentrate grades were higher than those obtained using 1,600-gram charges (table 4).
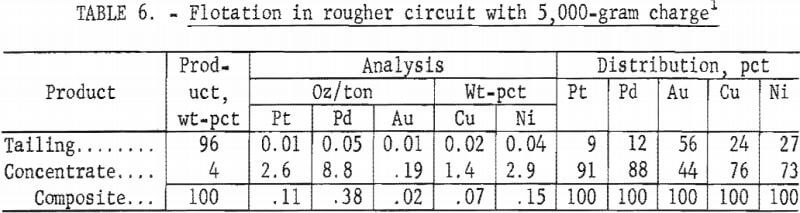
The tenor of a Stillwater rougher concentrate is compared in table 7 with values reported for a flotation cleaner concentrate produced at a Merensky Reef concentrator, Republic of South Africa. The considerably higher palladium-to-platinum ratio of the Stillwater concentrate compared to the Merensky concentrate was the result of the difference in tenor of the two ores.
X-ray diffraction analysis of the Stillwater rougher concentrate showed that approximately 50 vol-pct was comprised of the MgO-containing minerals, chlorite and talc, even though these minerals constitute only a small percentage of the ore. In an attempt to prepare a concentrate high in platinum and palladium values and low in gangue minerals, tests were made to investigate the use of a water-soluble polymer (Hercules Minflo I) gangue depressant in the rougher circuit. By using 0.06 lb Minflo I per ton of ore, concentrates containing 2.4 oz/ton Pt and 12.3 oz/ton Pd were prepared. Recoveries of platinum and palladium, however, were significantly lower, decreasing to 80 and 84 pct, respectively.
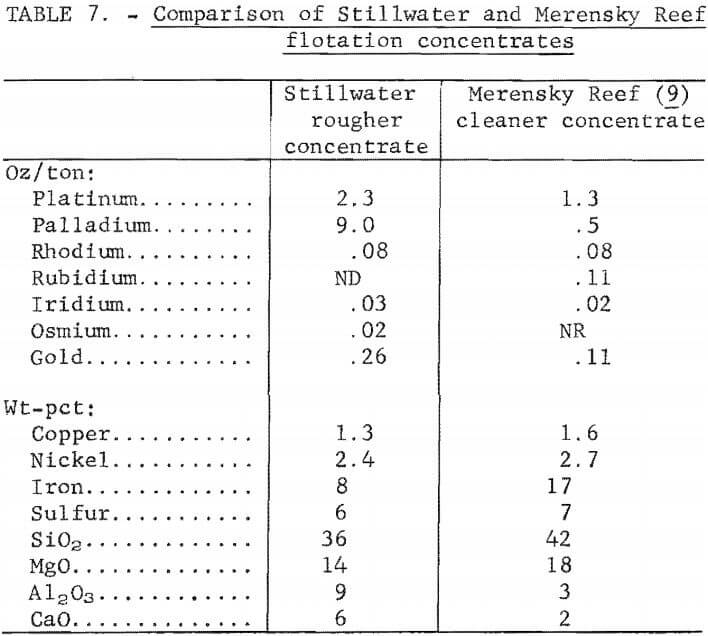
The analysis of a rougher tailing is shown in table 8. The material may be considered for use as a raw material for the manufacture of portland cement or a a source material for producing alumina.
Cleaning of Rougher Concentrates
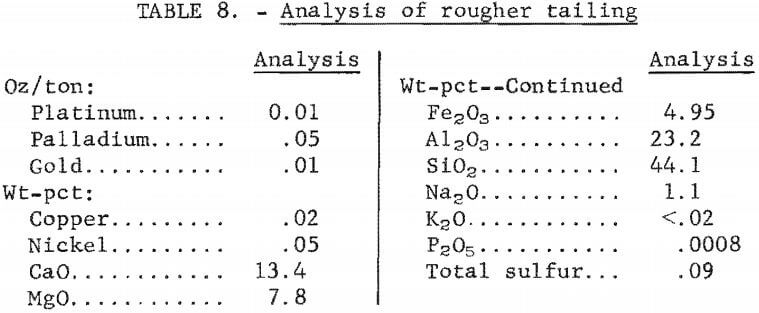
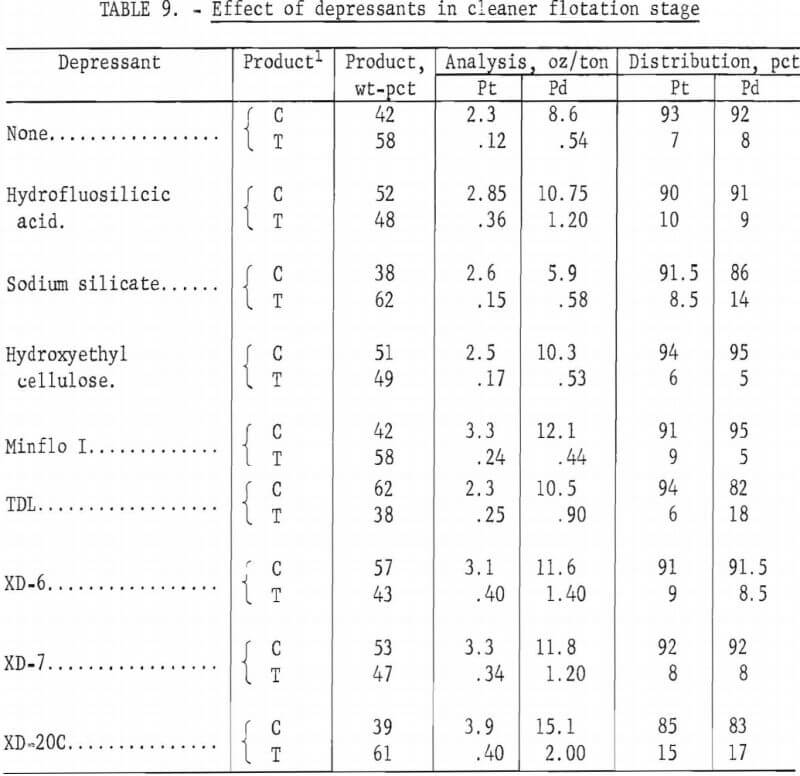
Because of the desirability of producing a concentrate high in platinum and palladium values for feed to a matte smelter or to a hydrometallurgical operation for extraction of the metal values, flotation tests employing a cleaner step were made. Rougher concentrates prepared under best conditions using the MBT-acid suite as previously described were fed to the cleaner cell. In preliminary tests, flotation was carried out at 6 pct solids and a pulp pH of 4. Wet concentrate from the rougher flotation circuit was repulped with water. No additional sulfuric acid or frother was added. The following reagents were investigated as depressants for the gangue minerals: hydro-fluosilicic acid, sodium silicate, hydroxyethyl cellulose, and water-soluble polymers Minflo I, TDL, XD-6, XD-7, and XD-20 (all Hercules products). The amount of depressant added was 0.3 lb/ton of rougher concentrate, except for hydrofluosilicic acid, where 4. 5 lb/ton were used. Conditioning and flotation times were 10 and 5 minutes, respectively. Results are shown in table 9. Comparable Pt-Pd recoveries and concentrate grades were obtained for tests using Minflo I, XD-6, and XD-7 depressants. The cleaner tailing was markedly lower in palladium content when Minflo I was the depressant.
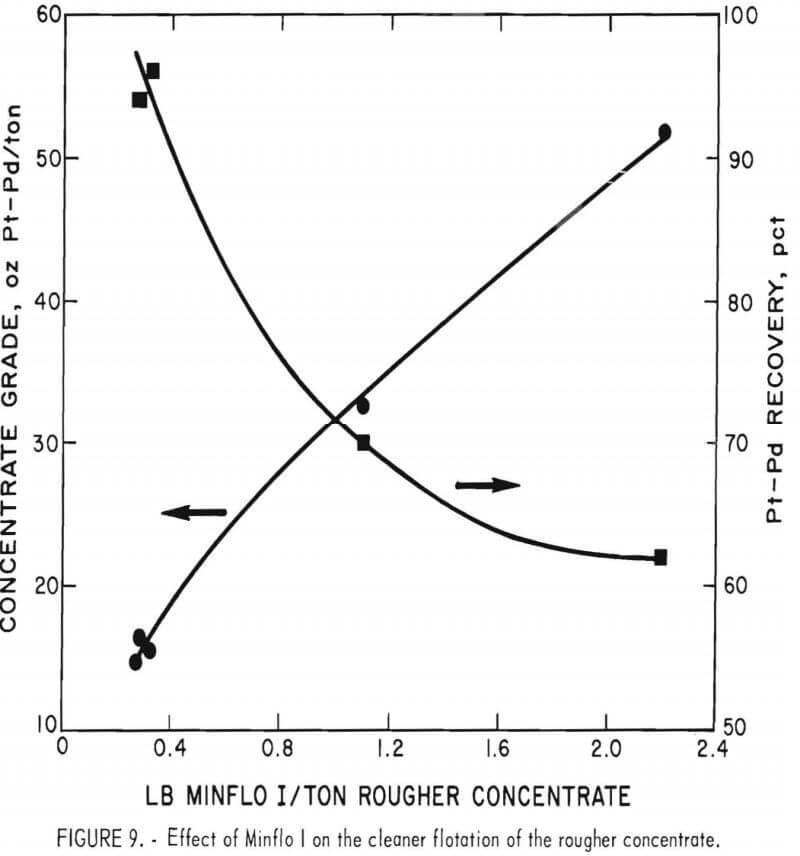
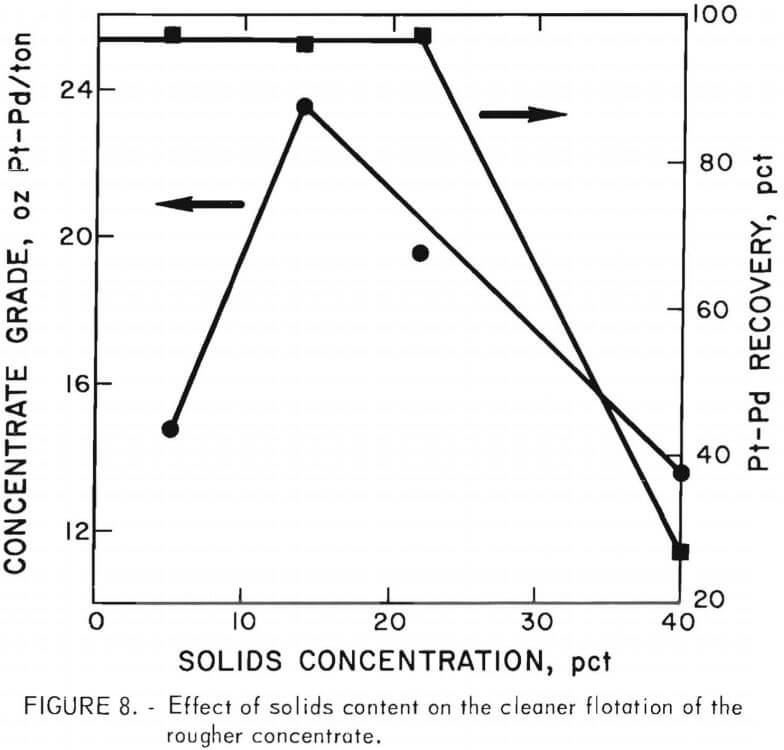 Since the preliminary test results were encouraging, additional tests using Minflo I in the cleaner circuit were made. The effects of solids content of slurry and the amount of Minflo I added were evaluated. Other conditions were the same as those used in the preliminary tests. Results are shown in figures 8 and 9. A 14-pct-solids slurry and an addition of 0.3 lb Minflo I per ton of rougher concentrate yielded the best results. Flotation at solids content of more than 15 pct resulted in a marked decrease in concentrate grade. Increasing the amount of Minflo I in the cleaner circuit from 0.3 to 1.0 lb/ton of rougher concentrate resulted in an increase in the grade of cleaner concentrate from 4.9 oz/ ton Pt and 11.5 oz/ton Pd to 8.0 oz/ton Pt and 24.0 oz/ton Pd. Recovery of platinum and palladium values decreased from more than 96 pct to approximately 70 pct.
Since the preliminary test results were encouraging, additional tests using Minflo I in the cleaner circuit were made. The effects of solids content of slurry and the amount of Minflo I added were evaluated. Other conditions were the same as those used in the preliminary tests. Results are shown in figures 8 and 9. A 14-pct-solids slurry and an addition of 0.3 lb Minflo I per ton of rougher concentrate yielded the best results. Flotation at solids content of more than 15 pct resulted in a marked decrease in concentrate grade. Increasing the amount of Minflo I in the cleaner circuit from 0.3 to 1.0 lb/ton of rougher concentrate resulted in an increase in the grade of cleaner concentrate from 4.9 oz/ ton Pt and 11.5 oz/ton Pd to 8.0 oz/ton Pt and 24.0 oz/ton Pd. Recovery of platinum and palladium values decreased from more than 96 pct to approximately 70 pct.
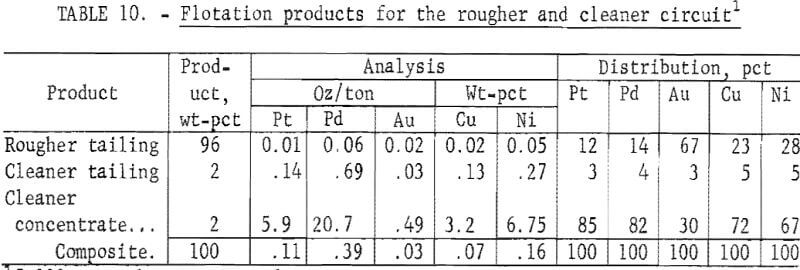
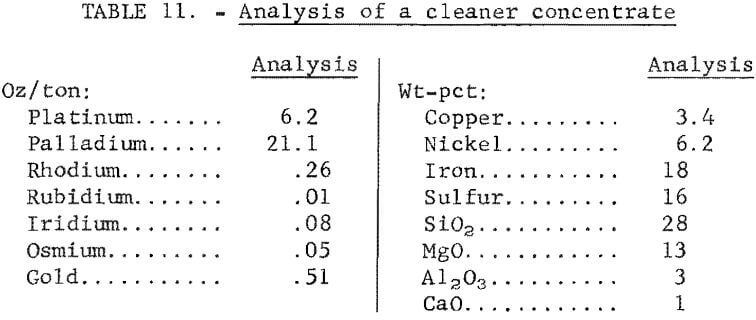
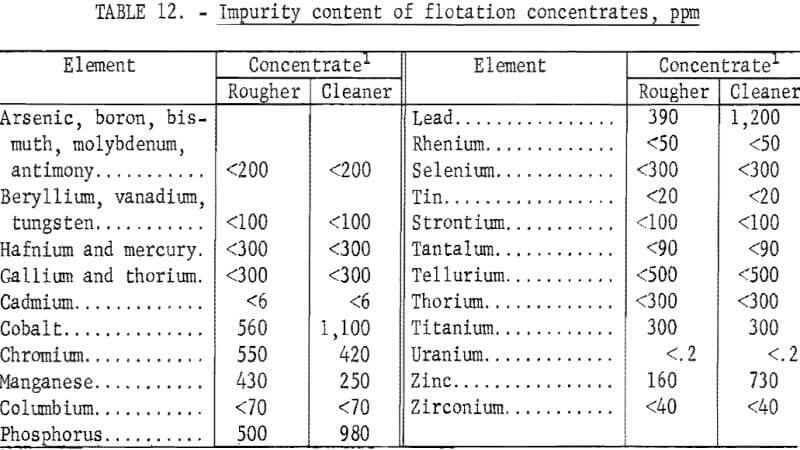
The best flotation conditions for both rougher and cleaner flotation stages were applied to 5,000-gram charges of ore. The results of a typical flotation test are given in table 10. A detailed analysis of a cleaner concentrate is given in table 11. The platinum-group metal content was more than twice that of a rougher concentrate, and the Al2O3 and CaO contents were significantly lower (see table 7). Because flotation concentrates would be subsequently treated by smelting-leaching or direct leaching techniques to recover platinum-group metals, impurity contents are an important consideration. In table 12, the impurity contents of a cleaner concentrate are compared with those obtained in a rougher concentrate. The data show that the impurity levels in both concentrates are low.
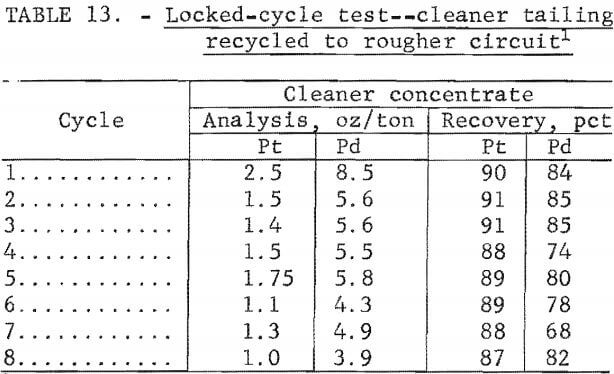
Locked-cycle flotation tests using 1,600-gram charges were made to determine the cumulative effects of recycling cleaner tailing to the rougher circuit on concentrate grade and platinum and palladium recovery. Results of an eight-cycle locked test (table 13) showed a decrease in platinum and palladium recovery and a decrease in concentrate grade as the circulating load of cleaner tailing increased.
Platinum – Palladium Flotation Summary
A flotation method using mercaptobenzothiazole collector in an acid circuit was devised for preparing a platinum-palladium concentrate from Stillwater complex gabbro (Montana). Bench-scale experiments showed that rougher flotation recovered 91 pct of the platinum and 88 pct of the palladium in a concentrate assaying 2.6 oz/ton Pt and 8.8 oz/ton Pd. When the rougher concentrate was cleaned, a cleaner concentrate was obtained that had more than twice the Pt-Pd content of the rougher concentrate and about 55 times the Pt-Pd content of the original ore. About 7 pct of the platinum and palladium values remained in the cleaner tailing. If the higher grade concentrate is desired, the platinum and palladium values in the cleaner tailing must either be discarded or recovered by treating the tailing outside the milling cycle.
As part of its mission to develop technology that will help increase the supply of critical and strategic minerals from domestic resources, the Bureau of Mines has investigated methods for beneficiating platinum-group metal ores from the Stillwater complex, Montana. This report presents the result of a bench-scale study employing froth flotation to recover a sulfide concentrate containing platinum-palladium values from a mineralized gabbro. Best results were obtained with a flotation scheme utilizing a mercaptobenzothiazone collector and sulfuric acid. Rougher concentrates containing approximately 11 oz Pt-Pd/ton and 90 pct of the metal values in the ore were obtained. Subsequently, cleaner concentrates containing approximately 26 oz Pt-Pd/ton and between 80 to 85 pct of the Pt-Pd values in the ore were prepared.
