Table of Contents
Metallurgical research has discovered many an alloy possessing properties not combined in any single metal, and progress still consists chiefly in the investigation and utilization of alloys. In the case of iron, the demands of automobiles, high-speed machines and high-duty engines have led to the production of special iron alloys which will meet any reasonable specifications in that field. In like manner, the bronzes, brasses and other alloys of copper have been brought to remarkable perfection, and for nearly every industrial purpose some alloy has been found more suitable than the pure metal.
Less complete success has attended the attempt to find substitutes for gold, platinum, and the other precious metals. Indeed, it is not likely that a material can be produced which will possess all the properties of any one of them; yet it is reasonable to hope that, for any given use, the properties required may be found in some less expensive material. Thus, in incandescent electric lamps, the wire passing through the thick glass neck of the bulb was, until recently, almost universally made of platinum, for the single reason that no other known material, suitable as a conductor, had the same coefficient of expansion as glass. But a comparatively recent investigation of the iron-nickel series has shown that alloys of those metals maybe produced, the coefficient of expansion of which can be accurately controlled between that of iron or nickel and zero. Thus, in that particular industry, a substitute for platinum has been found.
According to statistical reports, not only considerable quantities of gold and iridium, but also more than one-third of the annual supply of platinum, are used (and, in the nature of the case, irrevocably lost) by dentists. Platinum is thus-employed in several forms. As a thin foil, it serves various purposes for which its high melting point, pliability, chemical resistance, and other properties—including the ease with which it may be soldered—are invaluable. But it is most extensively used in the alloy with iridium, which is more resistant chemically than pure platinum, solders as readily, and possesses, besides, the quality of stiffness, and is not seriously softened by annealing at ordinary soldering temperatures. These advantages dictate its use, in spite of its high cost. The discovery of a substitute in dentistry for platinum and platinum-iridium, especially if it possessed useful properties which they lack, would be eagerly welcomed, and would find wide application in other arts also. The solution of this problem has been undertaken by the Research Foundation of the National Dental Association, for which the work described in this paper was done by the writer.
The substitute desired must satisfy the following conditions:
- Its melting point must be high, at least well above 1,200°C.
- It must not be affected by those chemical compounds formed in its application, nor should it oxidize at a soldering temperature.
- It must possess sufficient strength to resist stresses tending to change its form while in place, and at the same time be sufficiently pliable to be worked to the desired shape.
- Its coefficient of expansion must be low, in order that desired dimensions may be easily produced in the finished product. (This factor is important, since the range through which this material is manipulated is often more than 1,000°C.)
- It should unite readily with gold, silver and similar metals, and their solders.
- Its cost of production should be low, as compared with that of platinum.
After the entire list of metals had been considered with respect to these conditions, it was evident that any search for the desired material must be among the alloys, since experience has shown that the physical properties of a metal may be radically changed by the addition of varying amounts of another element, or of several elements, as in the case of steels, brasses and bronzes.
Considerations based upon the periodic law of atomic weights, and the table constructed in accordance therewith by Mendeleeff and Lothar Meyer, led to the conclusion that the field for profitable research was narrowed to the elements chrome, manganese, iron, cobalt, nickel, copper, silver, palladium, gold, molybdenum and tungsten, and their alloys (ruthenium, iridium and osmium, likewise theoretically indicated, being ignored because of their costliness and scarcity).
When two or more metals are brought together in the liquid state, they mix exactly like two ordinary liquids. When the temperature is lowered the solidified mass may contain any one of the four following constituents: Pure components; solid solutions; compounds; and eutectics—or some combination of these.
A comparison of the properties of different alloys containing these constituents has shown that they impart their characteristic properties to the alloy of which they form a part; in fact, the relation between the constitution of an alloy and its mechanical properties is so clearly defined that the possibilities of industrial application may be predicted for a given alloy, if its constituents are definitely known. Conversely, if a certain application is desired, as in the problem under consideration, a definite limit may be placed upon the number and amount of constituents permissible.
Fortunately, the number of constituents is limited to four, as given above. Pure metals impart their own characteristics; solid solutions are, in general, the ductile constituents (if formed of ductile metals or of a preponderance of one ductile metal); compounds and (usually) eutectics are hard and brittle, while the latter, even when present in very small amounts, tend to solidify between the grains of the alloy, thus destroying its ductility.
The problem was to determine what combination, if any, of the elements named, in whatever form, would meet the assumed set of specifications.
Accordingly, each of the 11 elements was combined, in varying proportions, with each of the other 10, giving 55 binary series to be considered with regard to their suitability as practical substitutes for platinum and platinum-iridium alloys.
Work on Alloys made by Fusion
Behavior under the hammer, or when drawn through draw plates, and under the influence of acids and alkalies, was sufficient to indicate whether any particular specimen should be discarded at once or made the subject of further investigation.
It was not considered necessary to measure the various melting temperatures encountered, as only a complete fusion, free from all contaminants, was desired. The purity of all components was the highest obtainable, while the purity of the resulting alloys was checked by microscopical and, if necessary, by chemical analysis.
Apparatus
The Gran-Annular electric furnace was used in this set of experiments, and served as no other type of furnace would have done under conditions involving constant use, quick heating and cooling, sensitive control, and low upkeep cost. This is of the granular-carbon resistance type, in which temperatures are limited only by the melting point of the
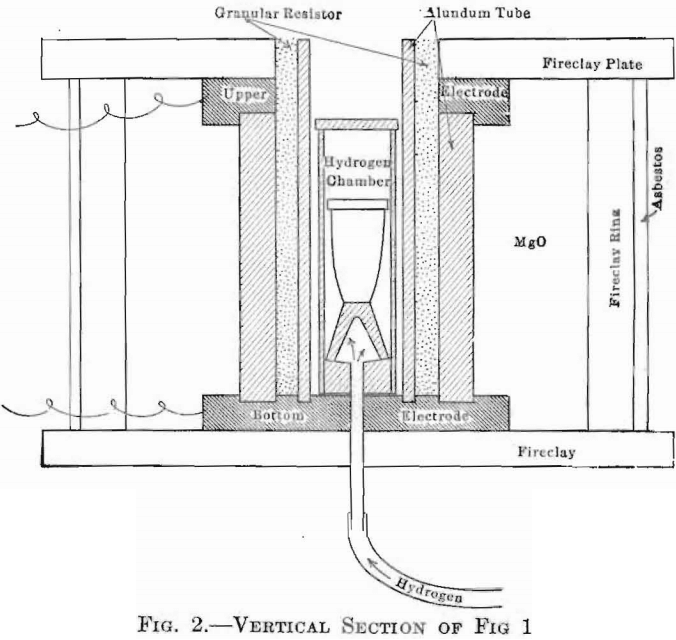
tubes used. With tubes of alundum, it can be safely run up to 1,800°C. This furnace is shown in Figs. 1 and 2, the latter a vertical section, showing also the means adopted for introducing any desired atmosphere into the crucible chamber. This latter device consists of a cylindrical alundum crucible, fitted with a Marquard tube which passes through the bottom, of the furnace and thence to the generator, provided with close-fitting cover, so that a gas may be maintained within it, and surrounding the crucible containing the melt, at a pressure sufficient to cause an outward flow through all the pores of the chamber walls, thus effectually preventing contamination by the atmosphere of the outer furnace chamber.
When this furnace is run above 1,200°C., the atmosphere within the heating chamber is of the following analysis: 0, 0.20 per cent.; N, 68.90; CO2, 0.70; CO, 30.20 per cent.
The tests for the general properties of the alloys, such as malleability, ductility, corrodibility, etc., are so simple as to require ordinary laboratory appliances only.
Table I gives a list of crucible materials, protective atmospheres, and covers, which have been found satisfactory in fusing these metals.
Results
Some of the 55 binary series, as, for instance, those high in iron, manganese, or chromium, indicated at once their inability to meet specifications, while others, as those of nickel-palladium, nickel-tungsten, etc., required careful tests, and many degrees of concentration, to determine their comparative properties.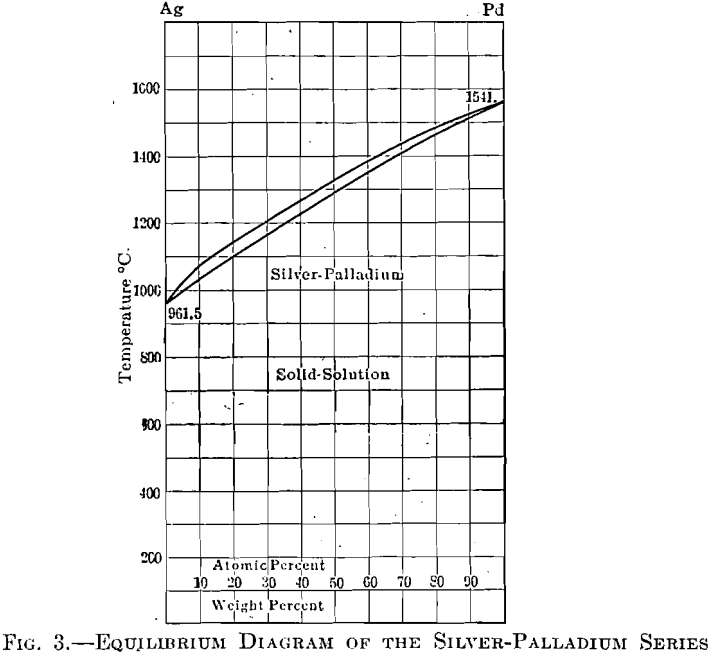
The investigation of these series (with the exception of those high in tungsten or molybdenum, which are not adaptable to fusion methods) is represented by nearly a thousand melts, which are unequally distributed among the different series, the number of fusions necessary in each series depending upon how nearly it approached the standard specifications.
The numerous negative results are not reported here, because they would greatly increase the volume, without correspondingly adding to the value, of this paper. It is sufficient to say that of these 55 series, only those of palladium with gold and silver have proved practically valuable.
The equilibrium diagram, Fig. 3, of the silver-palladium series shows a complete series of solid solutions. Alloys of these, in any proportions, have been found to be very soft, chemically inert, and if chosen of a composition to give a sufficiently high melting point, meet the necessary requirements of a foil to replace platinum, in the field of dentistry. The cost of this material will range from about 1 to 50 per cent, of that of platinum, depending upon the melting point desired.
The palladium-gold alloys (Fig. 4), however, are superior to those of silver-palladium, as gold is superior to silver. As in the palladium-silver series, the range between liquid and solid is very narrow, resulting in little tendency toward segregation. Fig. 5 shows curves having melting points as ordinates and cost per cubic centimeter, of silver-palladium and gold- palladium alloys, as abscissae.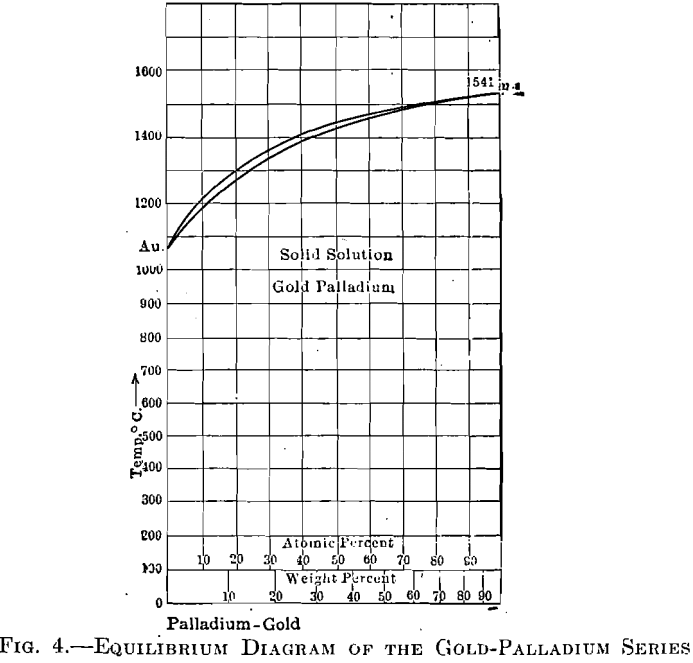
Metals are usually price-listed by weight; consequently an erroneous idea is generally prevalent as to the actual relative cost of different available materials, when applied to a given operation. A certain vessel, for instance, will require a definite volume of material, irrespective of its weight, which depends upon its density. Suppose, for example, that the volume of this vessel be 1 c.c. To fill this with platinum will cost, at present quotations, about $30.50; with gold, $12.50; and with palladium, about $22. This is not the popular conception of the relative cost of these elements which, when compared by weight, is gold, $20.67; platinum, $48; and palladium, about $60 per ounce.
In connection with this series is a phenomenon for which no explanation is evident; 1 per cent, of palladium darkens gold perceptibly; at from 2 to 3 per cent., the color is a dull light bronze; while at about 10 per cent., no trace of a yellow color can be detected.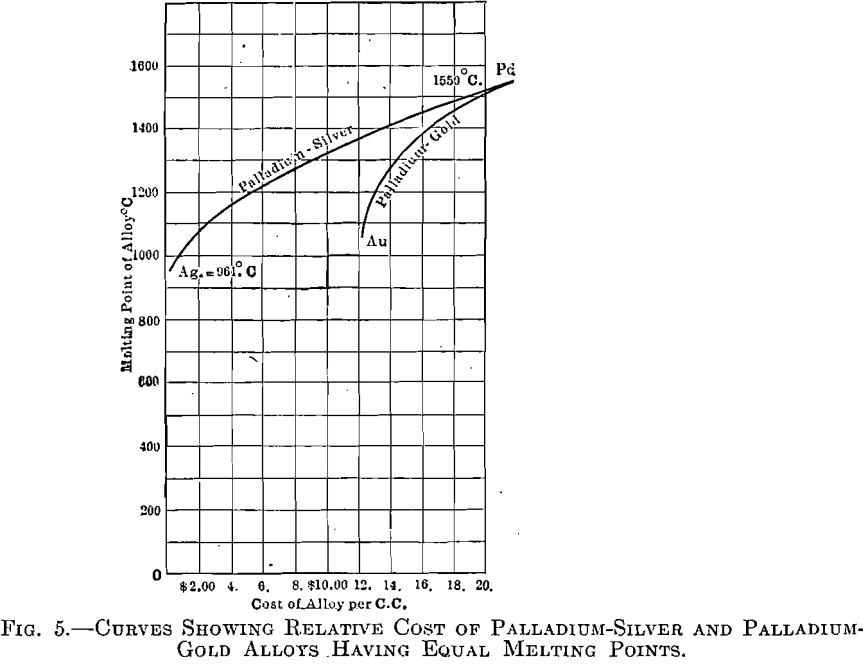
In the case of the silver-palladium alloys, a yellow tinge is perceptible throughout the series, which phenomenon is equally hard to explain, considering the pure whiteness of both components.
Although platinum, in its softer forms, as in foil, may be replaced by the above-described palladium alloys, investigation to this point had produced nothing in the nature of a hard, strong, non-oxidizable, non-corrodible material which would serve as a substitute for the platinum-iridium alloys.
https://www.911metallurgist.com/tungsten-molybdenum-alloys/
Practical Deductions
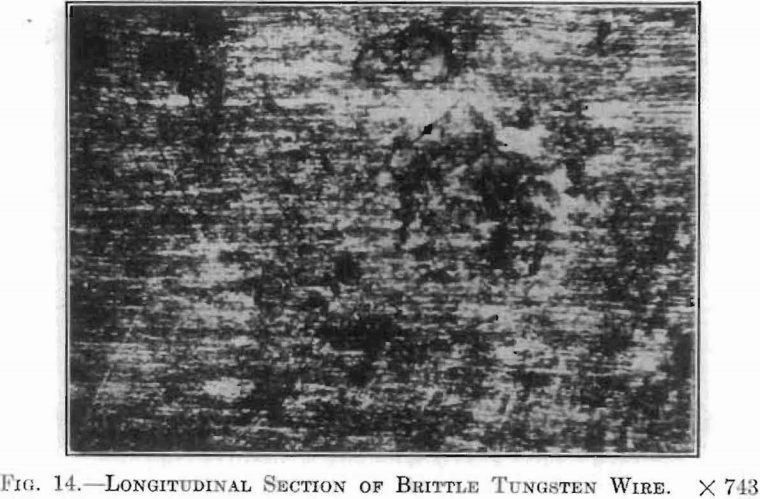
With the application of these crystallographic considerations, the probable cause for the difficulties encountered in the production of ductile tungsten, and to a greater extent, of ductile tungsten alloys, seemed apparent. Careful microscopical examination of many specimens of drawn wire submitted to this laboratory revealed in all eases that brittleness is accompanied by a difference in crystal structure. Fig. 10, already described, showed a longitudinal section of drawn tungsten wire, which was very tough and flexible. Fig. 14, a longitudinal section of a similar wire, which was exceedingly brittle, clearly shows the discontinuity of the drawn, fiber-like structure.
In discussing the internal structure of metals it was pointed out that a sufficiently high temperature would permit the complete crystallization of amorphous metal. The larger crystals of this mass may impress their orientation upon the molecules of smaller crystals, thus causing crystal growth; and mechanical distortion, above a certain temperature, will not greatly reduce this grain size.
In devising a method for producing tungsten and its alloys in a non-brittle form, these points were regarded as fundamental. The brittleness of the examined specimens of drawn tungsten was regarded as due to an insufficient breaking up of its crystalline structure. Hence, in producing this material, or its alloys, in ductile form, it would be necessary to eliminate excessive crystalline material; and in order to accomplish this, the following means suggested themselves, based upon deductions drawn from earlier conclusions regarding the behavior of worked metals in general.
- In order to produce a practical minimum of crystal size in the starting ingot, the treating temperature must be as low as possible.
- In order to decrease the amount of crystalline material, cold-working conditions must prevail.
- In order to introduce sufficient distortion, with its accompanying formation of the maximum amount of amorphous material, resulting in greater ductility in the larger sized wires, drawing should be commenced at an earlier stage in the reduction of the briquet, because this operation is much more effective in breaking up a crystalline structure than is swaging.
- For obvious reasons, the above operation should be controlled by microscopical examination. Internal structure, not temperature, is the treating criterion.
That these premises are fundamentally sound is shown by the following results of experimental work.
Experiments
The experimental work, which involved the application of the above considerations, and the results of which are outlined in this paper, included investigations of the following series: pure tungsten, tungsten containing 0.75 per cent. ThO2, pure molybdenum, tungsten-gold, tungsten-palladium, tungsten-molybdenum.
The method adopted comprised the compression of amorphous powder to briquet form; the heat treatment of the compressed material; and the forging of the resulting ingot.
The tungsten and molybdenum used in these experiments were obtained through the courtesy of the Cleveland branch of the General Electric Co., and were the chemically pure material used for the production of drawn wires.
The tungsten was of two batches, one containing 0.75 per cent, of ThO2 which is the material largely used for lamp filaments, while the

other was tungsten in which no trace of impurities could be detected. The gold and palladium were purified in this laboratory by several re-precipitations. The preparation of the amorphous material, from which briquets were made, will be described in connection with the different series.
https://www.911metallurgist.com/compressing-sample-powder-briquet/
Metallography
Grinding and Polishing
After heat treatment the ingots were made ready for microscopical examination. The preparation of specimens of this small size (about 0.5 by 0.5 by 1.0) cm. was rather a delicate operation, but the final surfaces secured were all that could be desired. Excellent apparatus was available for this purpose, while the three grades of alundum, F, XF, and 65F, were found to be sufficient for polishing all samples. Care was taken during these operations not to introduce surface conditions which would mask important internal phenomena.
Etching
For pure tungsten and molybdenum or their high-percentage alloys, the most satisfactory etching reagent was found to be concentrated hydrogen peroxide. It is necessary, however, to have this boiling while in contact with the specimen, otherwise it is quite inert. For the alloys containing a precious metal also, either hydrogen peroxide or aqua regia was used, depending upon whether the tungsten and molybdenum, or the precious metal was to be attacked.
Microscopy
For microscopical examination, there was available, from both Carl Zeiss and E. Leitz, a very complete equipment, the detailed description of which is not deemed necessary.
Results
In order to test the soundness of the premises upon which the experimental work was based, it was necessary that experiments should first be directed toward the production of workable masses of pure tungsten and molybdenum themselves, for, should this not be possible, it would be useless to extend the work to their alloys; and, conversely, should these pure metals yield to such systematic treatment, as based upon conclusions drawn from earlier study of the probable theoretical conditions involved, then the application of similar methods should meet with success in the treatment of their alloys.
It must be pointed out here that no treatment would overcome defects in these alloys due to chemical interactions, but that if, for instance, brittleness, as in the case of tungsten and molybdenum, is due to a purely physical condition, then a method based upon the above considerations should meet with success.
There are two fundamental critical points to be determined: First, the proper ingot-forming temperature; and second, the lowest possible
temperature at which this ingot will yield to mechanical working. This latter point, must obviously be below the hot-working range.
With the above considerations in mind, and in accordance with the principles laid down in the preceding pages, the experimental work has been carried out, with results as shown briefly under the following separate heads:
- Pure tungsten;
- pure tungsten plug 0.75 per cent. ThO2;
- pure molybdenum;
- tungsten-gold series;
- tungsten-palladium series;
- tungsten-molybdenum series.
Pure Tungsten
The pure tungsten briquets of this series were made under a pressure of 14,500 kg. per square centimeter (about 200,000 lb. per square inch). The specific gravity of the resulting briquet was 13, taken as an average of nine specimens. This was determined from micrometer measurements of volume, and weight of the specimen, and represents about 30 per cent, of void in the briquet.
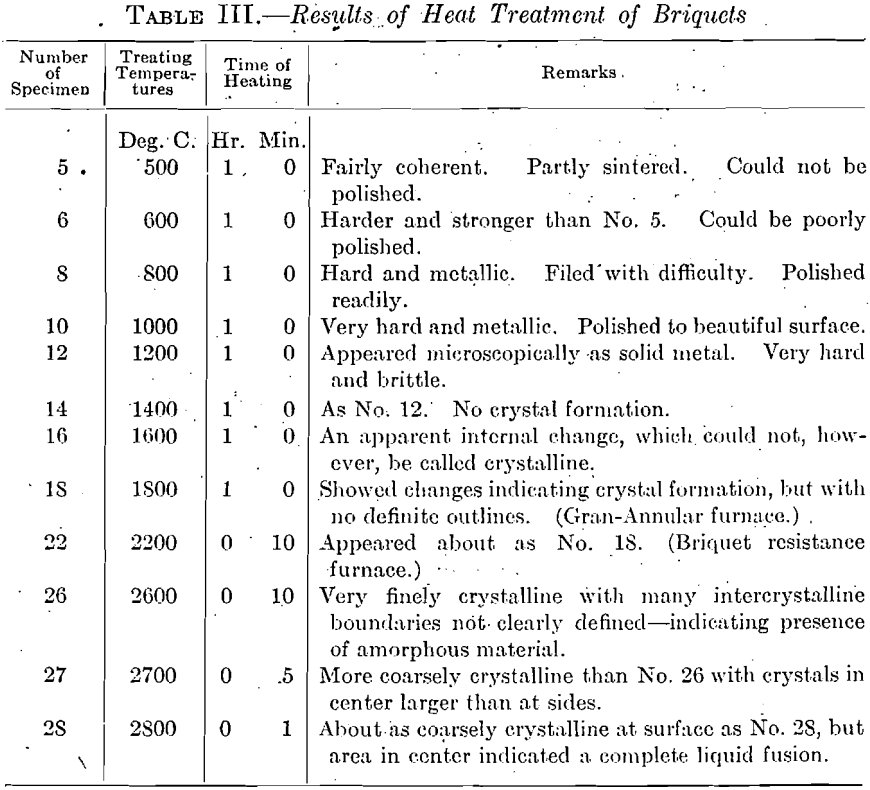
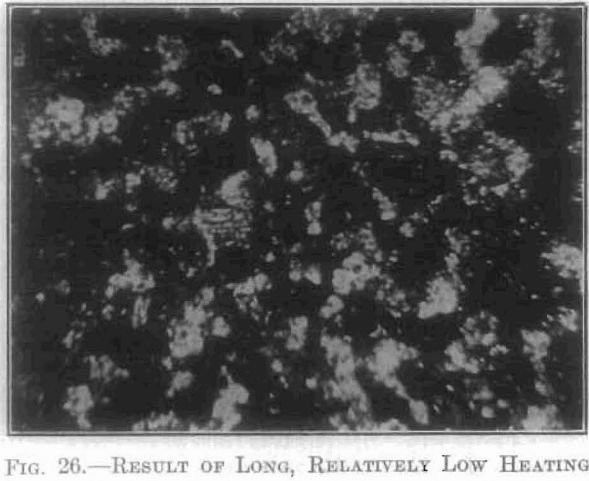
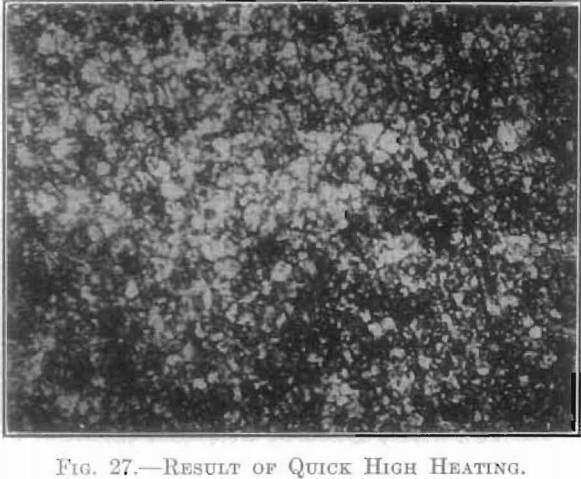
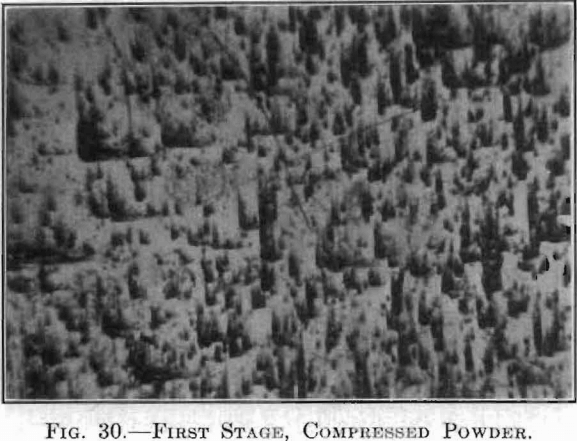
The extent and type of crystallization were found to depend upon the time-temperature factor. Prolonged heating at relatively low temperatures gave fewer and larger crystals, and crystals of different sizes (Fig. 26), while a flashing at higher temperatures (specimen No. 26) gave a very fine and uniform crystalline structure (Fig. 27), which was much to be preferred. In specimen No. 28 (Figs. 28 and 29), the heat of radiation from the sides of the briquet was so great that while the surface-temperature reading was only 2,800°C., the molten interior indicated a temperature above the melting point of tungsten (3,000°C.) at the center. Photomicrographs, Figs. 30 to 32, show the transition from the compressed powder to a solid, metallic, apparently non-crystalline mass, and finally to a solid, crystalline ingot.
The next step was to determine which of these stages of crystallization gave the best forging conditions. This was done by treating a series of each of these numbers at varying temperatures. It was found that, within certain limits, the lower the sintering temperature, the higher was the initial temperature necessary for forging.
No.5 could be forged at about 2,400°C.; No. 10 at about 2,200°C.; No.
18 at about 2,000°C.; No. 22, at about 1,700°C.; No. 26, at 1,275°C.; No. 27, at 1,350°C.; No. 28, at 1,375°C. These figures, of course, represent the initial welding temperature of the more or less powdery mass, the
necessary temperature falling at once when this welding had begun; they give no definite information other than indicating the necessity for at least partial crystallization in the treated ingot. Below these high temperatures the specimens were brittle in the first stages of forging, and if these temperatures were maintained after forging began, there resulted a marked crystal growth, instead of the desired reduction.
These series were represented by 92 forgings, or attempted forgings,

and the results gave a curve with a minimum forging temperature corresponding to a certain crystal size. This is represented approximately by No. 26 (micrograph Fig. 27). A higher temperature for a shorter time would give nearly the same conditions, or a lower temperature for a longer
period. It is thus seen that it would be useless to prescribe a definite treating temperature, or even one for forging, because the temperatures recorded were observed under rather restricted conditions, and in apparatus giving the worst possible conditions as to radiation, uniform heating, etc., due to the small scale on which the operations were carried out.
The results, however, showed how dependent are the physical properties of wrought tungsten upon the heat treatment and the crystallographic control exercised during the various stages of its manufacture.
The best specimens of this series were hammered out to a thickness of less than 0.5 mm. and, although it was necessary to increase greatly the heating current with increased cross-section of the ingot, the contact
resistance was enough to maintain a sufficiently high temperature. As the electrodes approached each other it became increasingly difficult to make accurate temperature measurements, since only the irregular outside edge of the flattened ingot could be sighted upon with the pyrometer.
No means were available with which to draw this material, so that its behavior during that operation, and the influence of the above-described control can only be assumed.
Tungsten with 0.75 per cent. Thorium Oxide.—An exactly similar series of experiments revealed only one point in which tungsten and thorium oxide reacted differently from the pure tungsten; but that point is of the greatest importance.
Sintering progressed with the gradual appearance of crystallites in a manner so closely paralleling the preceding case that the presentation of another almost identical set of figures and micrographs is thought unnecessary. But with respect to the crystal growth at higher temperatures, there was a marked difference. The presence of this small amount of thorium oxide was found largely to prevent a coarsely crystalline formation in highly overheated specimens; and even at stages just below the actual melting point the structure was very uniform. The explanation for this marked influence of thorium oxide is probably as follows:
Crystallization of this powdery, amorphous mass no doubt begins, as in the case of crystallization from the liquid state, at numerous centers, and proceeds, as in that state also, by the impressed orientation and resulting “absorption” of adjacent molecules of amorphous material, accompanied by an expulsion of foreign matter outward until, when neighboring crystals meet, the intercrystalline boundaries consist of thin sheets of this foreign matter. In most known cases the presence of this foreign material produces a weakness in the entire mass, but in the case of tungsten, the thorium oxide seems to be in such form as not only to prevent crystal growth, by its presence among the molecules of tungsten and later, between adjacent crystallites, but to maintain the strength of the entire mass. This may be a phenomenon similar to that which may be caused by iron oxide when two pieces of iron are welded together. It has been noticed; in this case, that no matter how. great the crystal growth occurring within either of the welded parts, no growth will extend across the weld; and this weld is usually the strongest section of the specimen.
Several experiments were performed, in which the thorium oxide was replaced by-other refractory oxides. Magnesium oxide, cerium oxide, and other refractory earths, produced a similar retarding action upon crystallization, but the effect, was not so marked. Although the experiments on other oxides than thoria were not sufficiently extended to locate these materials on a scale of their comparative effects in retarding the crystallization of tungsten and molybdenum, their value, for this purpose, seems to be a function of the temperatures at which they begin to soften.
The explanation of the accumulation of thorium oxide at the intercrystalline boundaries was first given by Zay Jeffries; and this phenomenon has been observed so often, during studies in connection with these experiments, that the logic of the explanation seems proved. Nothing is known regarding the physical characteristics of this rare earth when segregated under these conditions, but there is no reason for believing that it may not be in a vitreous, amorphous form, and hence, of great strength.
The influence of thorium oxide during forging was not so marked, but this is due to the fact that these experiments were carried out only to such extent as to prove that this material could be forged under the above condition. Indirectly, however, the effect of thorium oxide was present, in
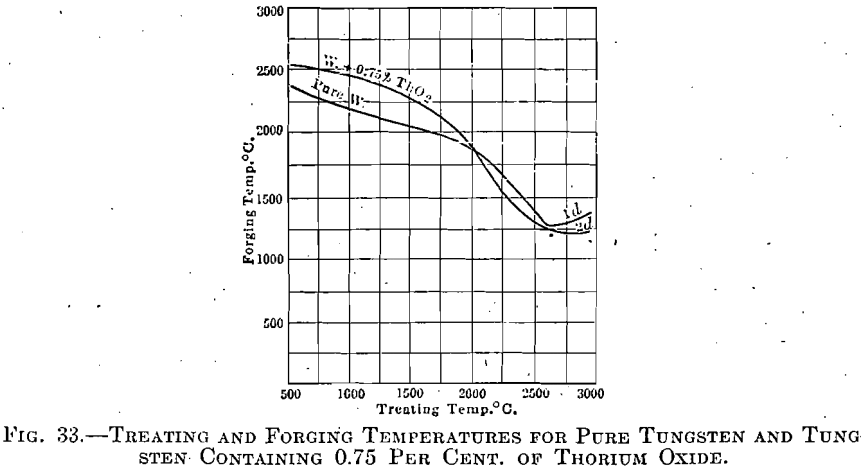
that those ingots formed at the higher temperatures, and in which excessive crystallization had been prevented by its presence, could be forged at the lower temperature, the curve not showing an upturned branch at the high-treating temperature end, as had that for pure tungsten (see Fig. 33).
Pure Molybdenum
The material used in this series, obtained from the General Electric Co., was the chemically pure powdered metal used in the manufacture of drawn wire.
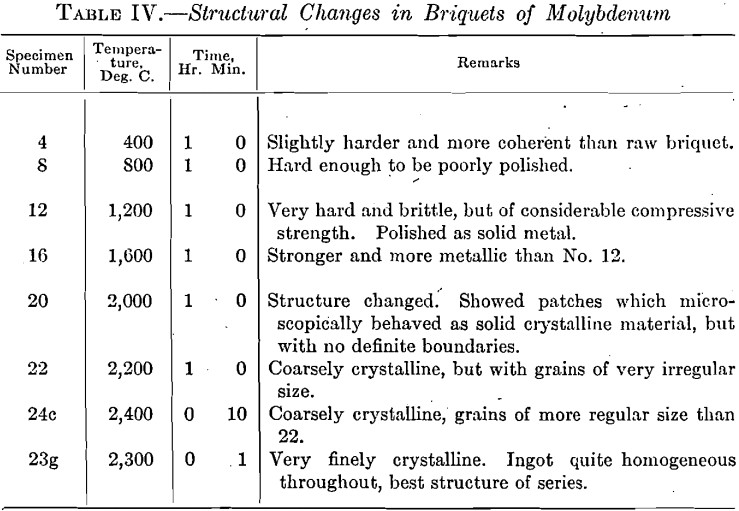
The raw briquets of molybdenum were made under a pressure of 14,500 kg. per square centimeter and gave an average specific gravity of 7.80. Table IV shows the change in internal structure of briquet with increasing temperatures.
The photomicrographs of this series could not be distinguished, in representing the transition from compressed amorphous powder to crystalline ingot, from those for pure tungsten, and hence are not given.
Specimen No. 23g, was of minimum grain size consistent with maximum crystalline formation. In this, as in the preceding series, it seemed necessary to reach a certain size of grain in order to produce an ingot free from patches of incompact amorphous material, the presence of which made it necessary to use a very high temperature at the beginning of the forging operation. On the other hand, too high a treating temperature produced a coarsely crystalline structure, with its inherent brittleness, which also could not be overcome, except at high temperatures.
The forging curve for pure molybdenum showed a minimum corresponding to No. 23g, with higher temperatures on either side, as in the case of pure tungsten.
The results of experiments on tungsten and molybdenum have given excellent support to the theory upon which the work was based, and furnished details of critical points which promise to be paralleled, and should be searched for, in any series of their alloys.
Having established the soundness of the principles involved when applied to these pure metals, the next step was to apply them in connection with their alloys.
It must be again pointed out that this method did not have as its object the production of true alloys, in the sense of those which solidify from the liquid state, but to avoid such consequences as might result from that condition, by welding together, at a temperature below the melting point, particles of molecular proportions, which should be as evenly distributed and uniformly mixed as are the molecules in a true solid solution.
Tungsten-Gold Alloys
To insure an intimate mixture of gold and tungsten the chemically pure tungsten metal was first oxidized and then saturated in an evaporating dish with an acid gold chloride solution containing the desired percentage of gold. This was carefully evaporated and dried, with constant stirring to prevent segregation; then transferred to an alundum boat and placed in the quartz-tube furnace for reduction under pure hydrogen. This material, when compressed into briquets, under 19,300 kg. per square centimeter (about 265,000 lb. per
square inch) pressure and examined under 2,500 diameters, did not show the slightest non-uniformity.
This series was investigated through a range from 0.1 to 20 per cent, of gold; but to give in detail the numerous negative results obtained would make this paper too cumbersome. A brief description of the behavior of these combinations in several proportions will suffice for the series.
No combination in this series could be forged at any temperature; even 0.1 (which was the smallest percentage of gold used) seemed to be sufficient to prevent working of the mass.
Below 1.4 per cent, the gold could not be detected as segregated at any stage of the treatment. This amount, however, was sufficient to cause coarse crystallization at about 2,500°C. Analysis showed the rather remarkable fact that very little of this gold was lost, even in those specimens which were treated to a temperature of 2,600°C., which is far above the accepted boiling point of gold.
The extreme fragility of these specimens at very high temperature prevented even an approximate determination of the melting point.
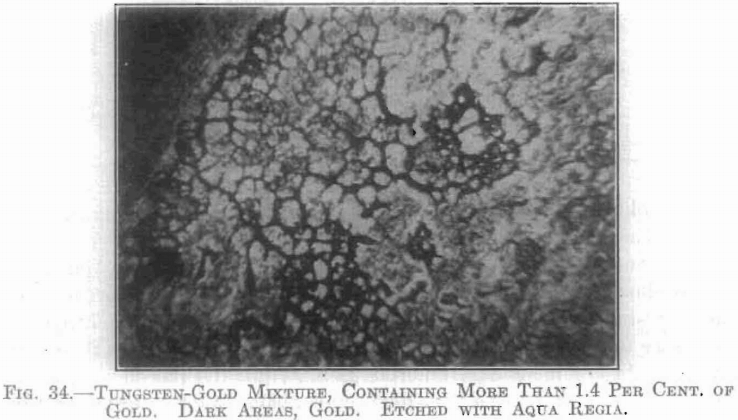
Above 1.4 per cent, of gold, a segregation resulted in those specimens treated between about 1,200°C. and 2,200°C. (see Fig. 34). Below about 1,200°C. no change could be detected microscopically, while above 2,200°C. a homogeneous crystalline mass resulted. An interesting phenomenon is noted in connection with temperatures above this latter point. In the case of specimens containing 5, 7, and 10 per cent, respectively of gold, after being heated to a temperature near 2,600°C. the gold content was found to be partly volatilized, and condensed in visible particles on the chamber walls and on the electrodes. In each case there remained a coarsely crystalline mass containing from 4 to 5 per cent, of gold.
No attempt was made to learn the nature of this material or to determine its properties, other than to prove its worthlessness from a mechanical point of view.
Tungsten-Palladium Alloys
A similar investigation of the tungsten-palladium series, but involving only 0.0 to 4.5 per cent, of palladium, gave results very similar to those of the preceding series. Below the melting point of palladium, no change could be detected. Above this point the mass became crystalline and possessed an inherent brittleness which could not be overcome. This was no doubt due to the fact that some reaction had taken place. Whether’a compound or solution was formed is not known, but that combination of some kind had occurred, was attested by the fact that the mass appeared homogeneous at all stages of the treatment under the highest magnification. .
These ingots of tungsten containing palladium were not found to be malleable, when worked under the above-described conditions.
Tungsten-Molybdenum Alloys
The tungsten-molybdenum series was investigated in various proportions of the components, taken at 10 per cent, intervals through the range from pure tungsten to pure molybdenum. The alloys represented by each of these intervals were found to be susceptible to certain conditions under which they could be wrought. The briquets were made under a pressure of 18,000 kg. per square centimeter and were then treated through a range of temperature in a manner similar to that employed in the previous series. Figures for the treating and forging temperatures of pure tungsten and molybdenum have already been given, while those for intermediate points are shown on the curves, Fig. 35.
The investigation of this series involved the treatment of more than 200 small ingots, and to give the details regarding each would be merely to repeat those already given for pure tungsten, which may be taken as
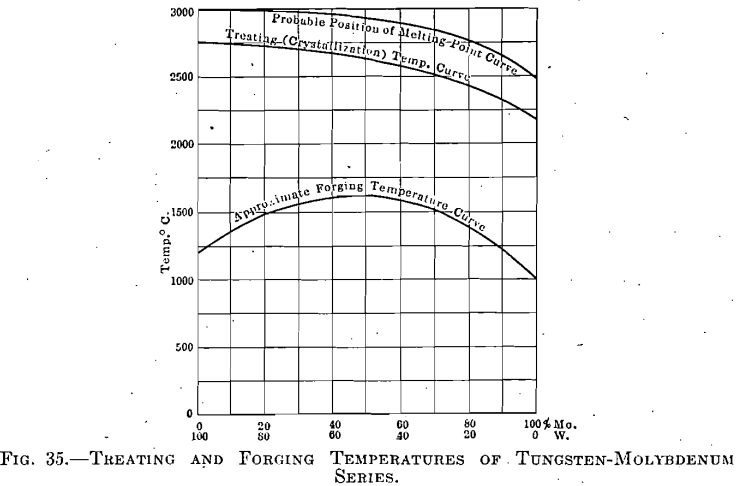
typical for any of these series. The formation of crystallites and crystal growth proceeds in a manner shown in the photomicrographs of the pure tungsten briquets, although—and here is an important point—the temperature representing a certain grain size changes with the relative amounts of the components, as the curve clearly shows. This is to be expected, and must be observed, in the treatment of the ingots; but it is not known whether this factor has received consideration in any attempted commercial production of such alloys.
A factor of equal importance is that of a corresponding change in the temperature of forging. If, for instance, only 1 per cent, of molybdenum were added to the tungsten, a treatment identical with that for pure tungsten would produce grains of such large size that even after, repeated forcing (and this requiring a higher relative temperature) the material would still be unreliable. On the other hand, the addition of small amounts of tungsten to molybdenum would necessitate higher treating and forging temperatures, as shown in Fig. 35.
Microscopical examination of these treated specimens revealed nothing that would indicate that these two metals formed other than a complete series of solid solutions; and if the crystallization curve may be taken as paralleling the melting-point curve (which assumption seems
not illogical), then this latter curve will be found, when determined, to occupy a position approximately as indicated in Fig. 35.
Fig. 36 shows a specimen of the 50 per cent, alloy as the raw briquet and after being forged; while Fig. 37 shows a microsection of this same specimen, after treating and before forging.
Summary and Conclusions
With regard to the degree of accuracy with which temperatures could be measured in these experiments; it must be pointed out that the object was not to establish these critical points for direct transference to any .commercial plant (for different types of apparatus would necessitate a determination of these conditions to suit each individual case), but to determine their existence and influence. It would also be of no avail to locate these critical ranges because every different set of apparatus and conditions would require a new standardization.
In these experiments, however, the temperatures necessary to produce a certain degree of crystallization were considered as being located with’a fair degree of accuracy, insofar as this may not be qualified by the existence of working conditions which were far from ideal.
As to the measurement of forging temperatures, no claim is made for more than close approximations, for this was properly not a one-man operation, and was performed by the writer with one eye to the optical pyrometer the other on the milliammeter scale; one hand on the pyrometer rheostats, the other using a hammer on the upper electrode; while the heating current was controlled by one foot on a lever regulating the transformer and rheostat.
The experimental work resolved itself into three parts, each being marked by a different method of attack, necessitated by limitations encountered as the work progressed under previously adopted methods.
The first part consisted of experiments on binary combinations of those of the metals which it was feasible to consider, and the melting, points of which lay within the limits of ordinary fusion methods. The results of these experiments, performed as indicated therein, lead to the conclusion that metals or alloys of metals outside of the precious-metal groups, are unsuitable as substitutes for platinum.
The gold and silver alloys of palladium have been found to be excellent substitutes for platinum in its softer forms, and while not so chemically resistant, fill all requirements where conditions are not too rigid.
The second part develops the fact that except in two respects, pure ductile tungsten, and, to a lesser degree, molybdenum, meet all of the specifications of a practical substitute for platinum and its alloys. These two defects are its ease of oxidation, and the difficulty with which it can be soldered; and they have been overcome by coating with a precious metal or alloy, the resulting material being in many ways far superior to platinum or its alloys.
This material has met with instant demand, is in many cases replacing the best platinum-iridium alloys, and permits the performance of work which has been impossible with the materials hitherto available.
The third part describes the theoretical and practical considerations involved in the manufacture of wrought tungsten and molybdenum, and gives results of the proper application of a similar method in the laboratory production of their alloys.
Wrought tungsten and molybdenum were produced on a laboratory scale, but no success attended the attempted production of alloys of tungsten with gold and palladium; while on the other hand, the alloys of the tungsten-molybdenum series were produced in wrought form. These operations were governed entirely by metallographic control, and their success suggests the possible application of a similar method in a treatment of such metals as iridium, tantalum, rhodium, osmium, etc., in combination with each other, or with tungsten or molybdenum, which may result in the production of alloys possessing properties far superior to those of any material now available.

