Table of Contents
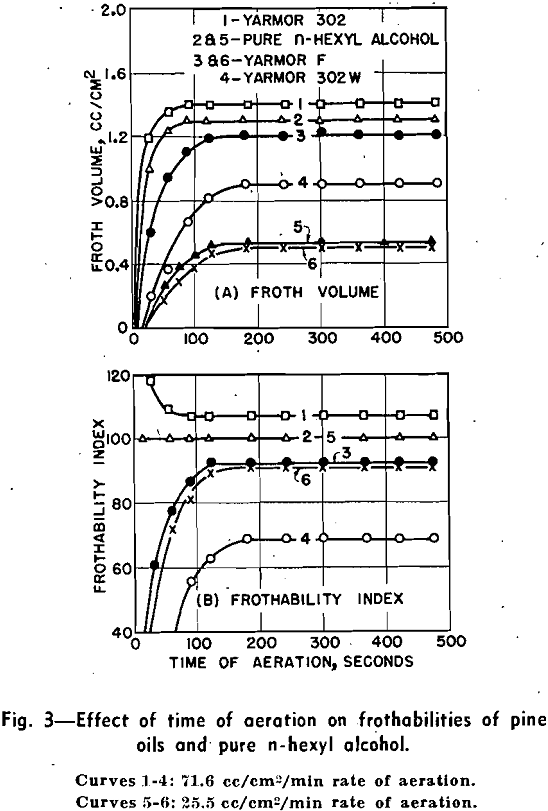
This paper presents the design and operation of a frothmeter capable of measuring the frothing characteristics of pine oils and other frothing reagents. The experimental data show that the frothability of pine oil is governed by: 1—rate of aeration, 2—time of aeration, 3—height of liquid column, 4—chemical composition of pine oil, 5—pH value of solution, 6—temperature of. solution, and 7—concentration of pine oil in solution.
Experimental Procedures
Except where otherwise stated, the data presented were established by means of the compressed air method. The volume and persistence of froth were recorded respectively at the end of 4 and 6 min of aeration at a constant rate of airflow of 29.3 cc per sec, which is equivalent to 71.6 .cc per sq cm per min, or 462.6 cc per sq in. per min. The aqueous solution for each test, containing 1000 cc of distilled water and 19.2 ± 0.5 mg frothing reagent, was adjusted to a pH of 6.9 ± 0.2.
Before each test, the glass cylinder, 13, was cleaned thoroughly with jets of tap water, ethyl alcohol, tap water, cleaning solution, tap water, and finally distilled water. The cylinder with stopcock,
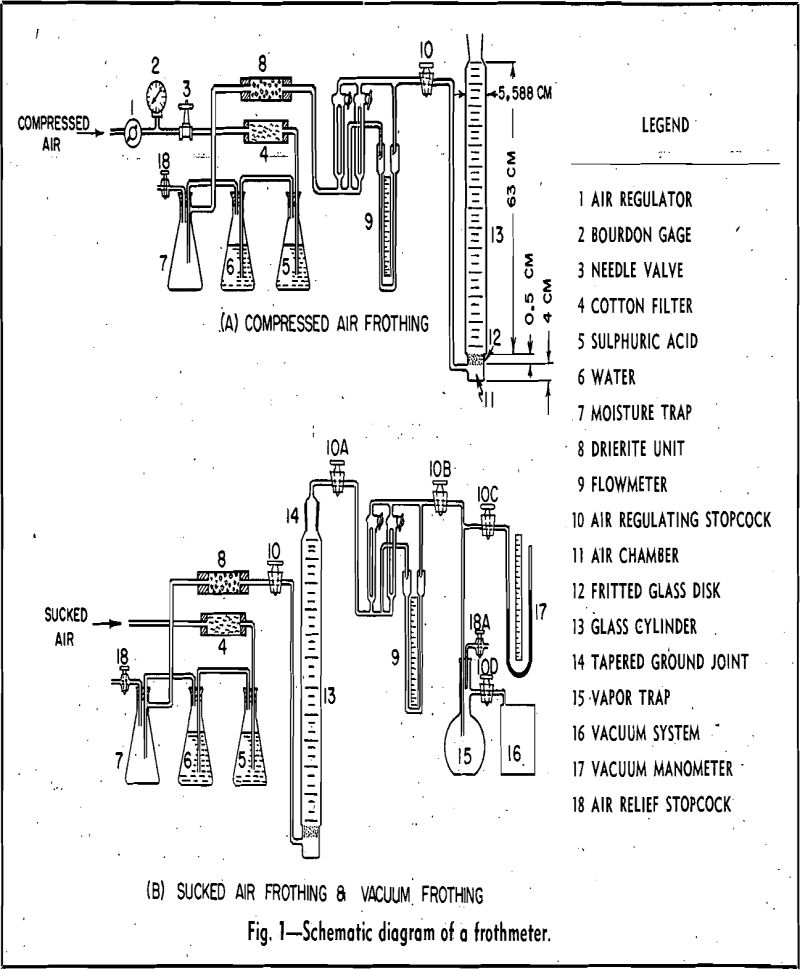
10, closed was connected to the flowmeter by rubber tubing. The cylinder was filled first with 1000 cc of distilled water, and a predetermined amount of frothing reagent was added. The pH of the distilled water was adjusted previously in a . glass beaker with solutions of sodium hydroxide and hydrochloric acid of cp grade. All pH values were determined by a Beckman pH meter. The upper , level of the solution was marked on the cylinder with a crayon pencil. Approximately 30 sec after the stream of compressed air was admitted into the purifying and drying system and the flowmeter, a moderate amount of air pressure was-built up to prevent water from dripping through the fritted glass disk, 12. The
stopcock, 10, of the cylinder then was opened. A predetermined rate of aeration was obtained by adjusting the needle valve, 3.
Frothability Index
The froth volume method is self-explanatory and has been used extensively in the field of froth flotation as well as foam industry. The frothability index method is proposed for the benefit of canceling the idiosyncrasies of the frothmeter employed.
The indices of the frothers were based on a standard frothing substance, pure n-hexyl alcohol. By testing a frother and the standard under the same
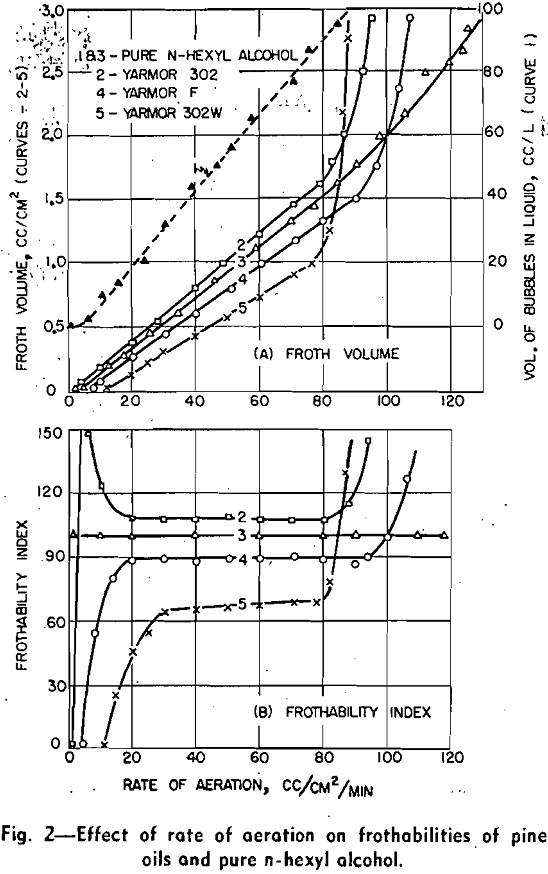
conditions and considering both the volume and persistence of the standard froth as 100, the indices of the frother were calculated from the following equations:
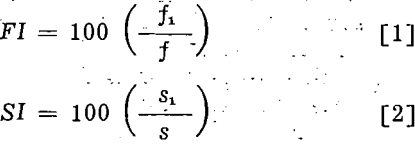
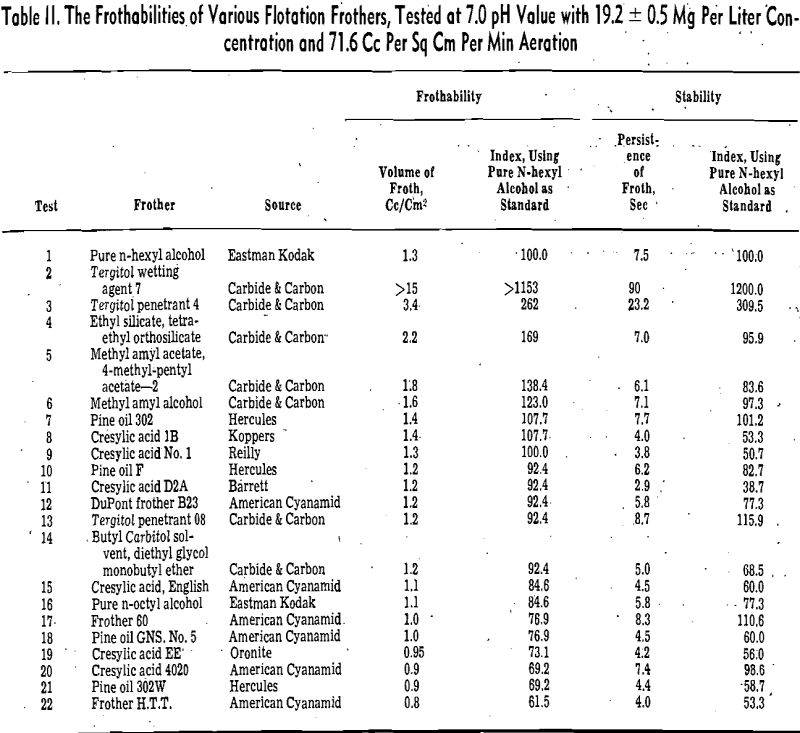
in which FI and SI are respectively the frothability and stability index of the tested frother, f in cc per sq cm, and s in seconds are respectively the volume and persistence of the standard froth, and f1 and s1 are respectively the volume and persistence of froth produced by the test frother.
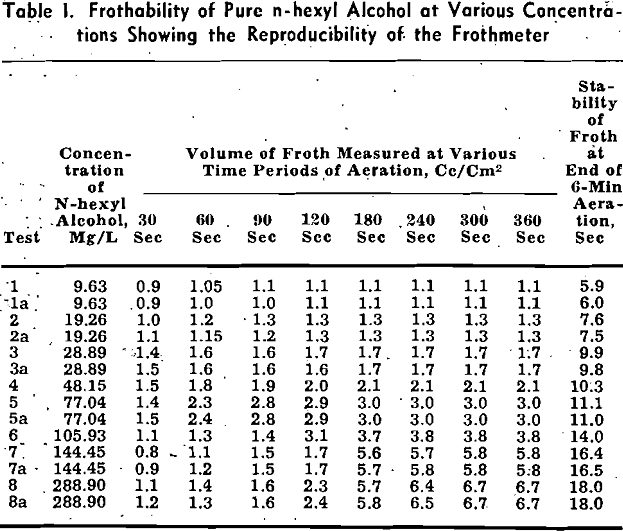
Pine oil is a complex mixture of terpene derivatives. It can be seen from this table that the frothing power of the constituents in neutral and acid solutions generally decreases in the following order: tertiary alcohols, secondary alcohols, ketones, ethers, and finally hydrocarbons. Since the chemical structure and the number of carbon atoms of the nonpolar groups of these constituents are somewhat similar, the difference of frothability is caused chiefly by the variation of the polar group and solubility. The double bond of the nonpolar group does not influence the frothability significantly.

Flotation with Pine Oil
To find how the relative frothabilities determined by the frothmeter will correlate with that in actual flotation, a Pittsburgh bituminous coal slurry was tested with different pine oils in a laboratory Fagergren machine.
The test data of vacuum frothing, not presented here, indicate that the effect of concentration and grade of pine oils on their frothabilities also can be detected by vacuum frothing. Vacuum frothing differs from both compressed air frothing and sucked air frothing in that the amount of air stored in the air-saturated liquid and in the empty space of glass cylinder is limited. After a short period of continuous frothing of small bubbles, the air is practically used up, and the remanent air is scant and capable only of providing intermittent large bubbles. The’ rate of degassing is directly proportional to the magnitude of the vacuum system. A high vacuum capable of quick degassing induces an earlier but shorter period of continuous frothing, as compared with a low vacuum.
