Table of Contents
This paper describes the production of cold or low temperature bonding method using sponge iron powder as a binder for iron bearing material after balling. Development of cold bonding pellet methods
Theoretical foundation of the cold bonding pellet method
The bonding mechanism of cold bonding pellets using sponge iron powder as a binder is based on the electrochemical corrosion and hydrolization according to electrochemical theory. The pellets are made of sponge iron powder (anode) and concentrate (cathode) with water. Due to the presence of electrolyte (water and atmosphere moisture), the current generated and numerous microgalvanic cells formed, which dissolved sponge iron powder, gave corrosion products that made the surrounding iron concentrate bonded.
The test showed that magnetite with maximum conductivity has the best bonding property, but the bonding property of hematite is not as good as that of magnetite. The bonding property of pyrite cinders depends on the chemical composition of mineral. Hydrated ores (such as limonite, etc.) with low conductivity have poor bonding property, therefore, they cannot be used to produce cold bonding pellets. Thus, the higher conductivity the iron ore concentrate has, the more dissolved sponge iron powder is in pellets and the pellets have better bonding property. Generally speaking, the amount of sponge iron powder added and its fineness, time of cold bonding, moisture, temperature and pressure are all related to the strength of the pellets. During the tests, moisture of 7-15%, higher atmospheric humidity and oxidation temperature of 40-60°C were kept depending on the type of iron concentrate, so that satisfactory bonding effect of pellets was obtained. Sufficient amount of oxygen also has a considerable effect on corrosion. As to the effect of oxygen in atmosphere, on one hand,oxygen can prevent FeO from further changing into ionic form when iron is transformed into solution of FeO, therefore, transformation of ferrous iron into ferric iron promotes further solution of iron. On the other hand, hydrogen, evolved from cathodic region, combines with oxygen, makes the cathodic region depolarized and speeds up corrosion process. Increase in pressure can also speed up corrosion. The influence of pressure on corrosion rate is obvious especially during depolarization process of oxygen.
The rusting process of sponge iron can be expressed as follows:
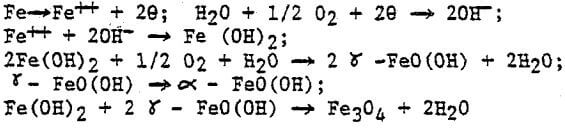
Study of cold bonding pellets in laboratory
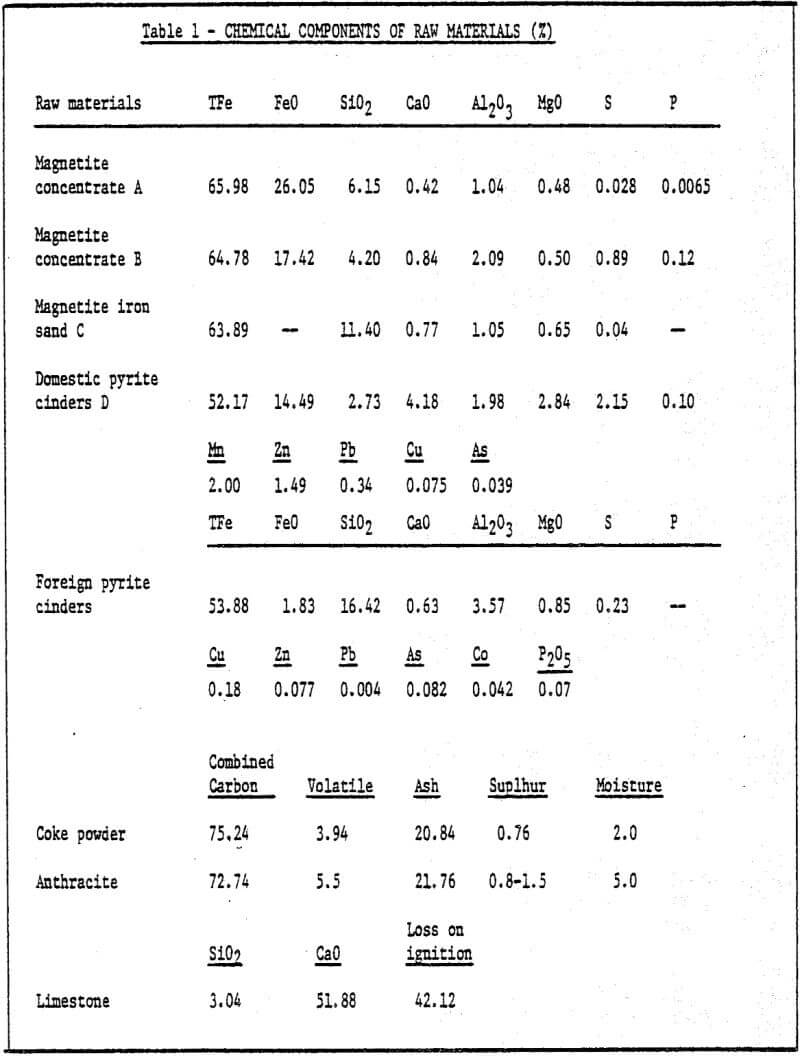
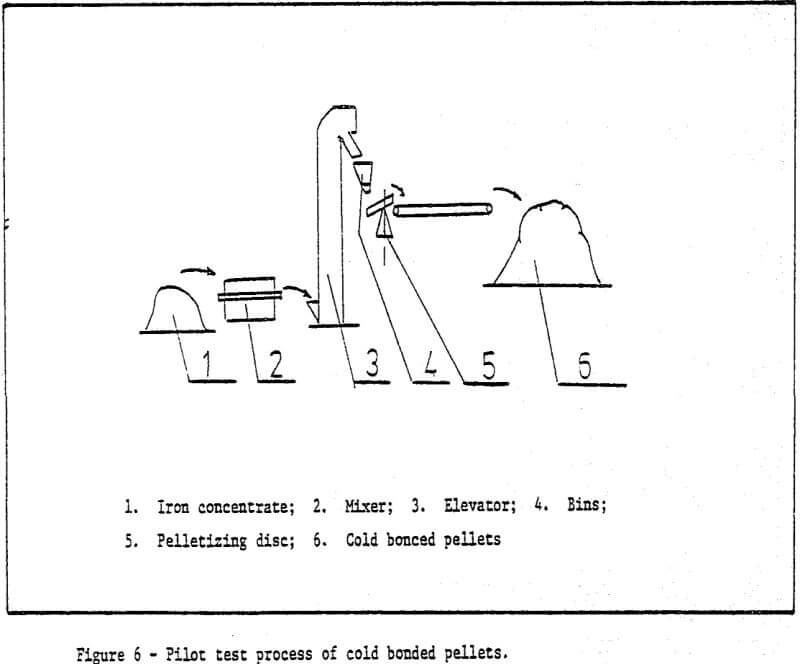
1) Raw materials – iron ore ground to desired size (85% -200 mesh) is pelletized by a pelletizing disc with a diameter of 1000mm. The chemical components of raw materials used are shown in Table 1.
2) Results of experiments.
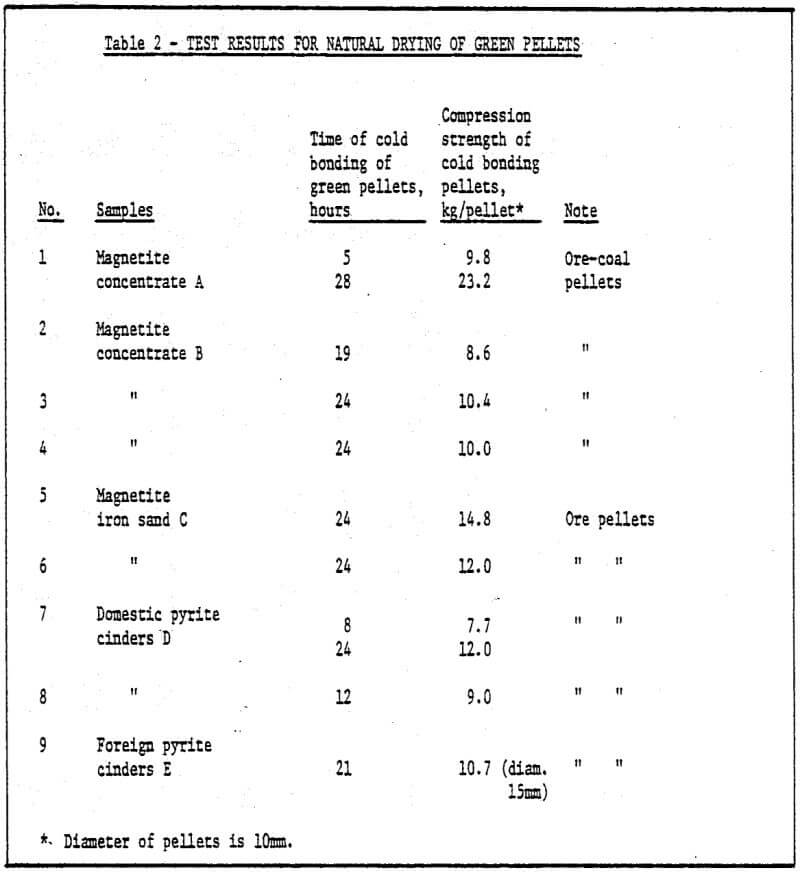
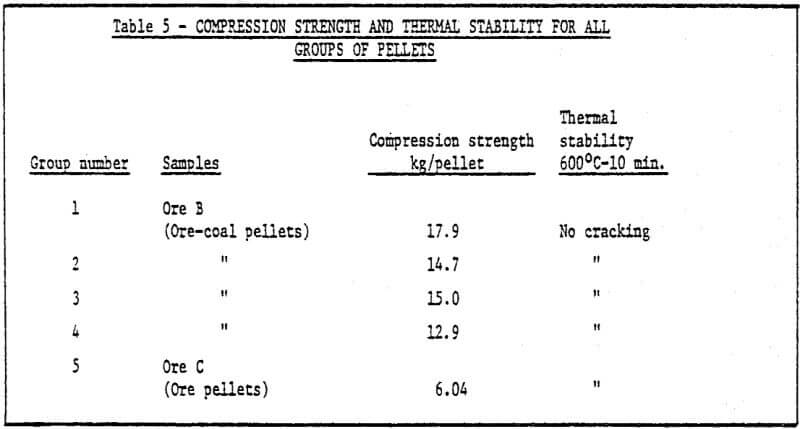
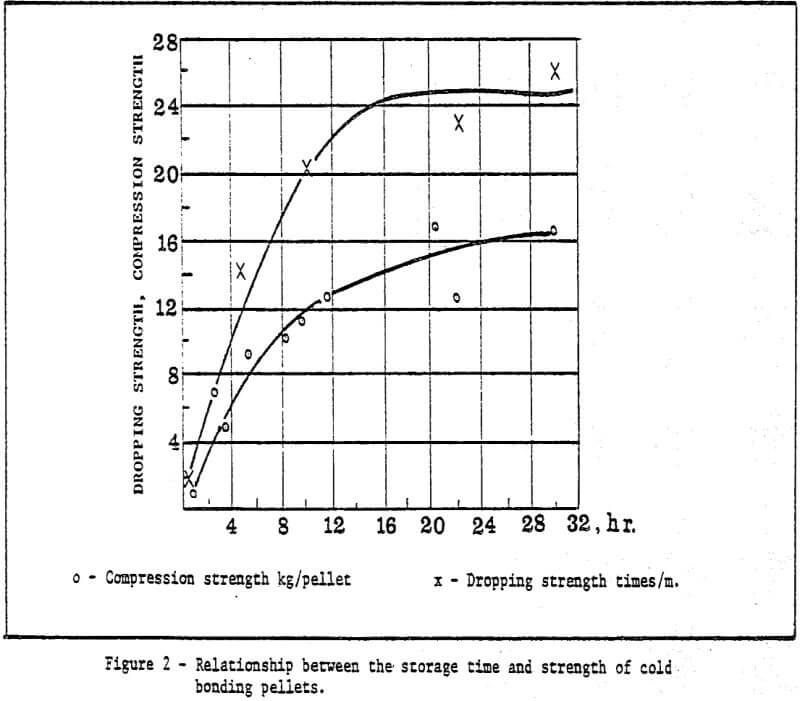
a) Storage method of green pellets – Green pellets reach the desired strength after 24 hrs. natural drying (shown in Table 2).
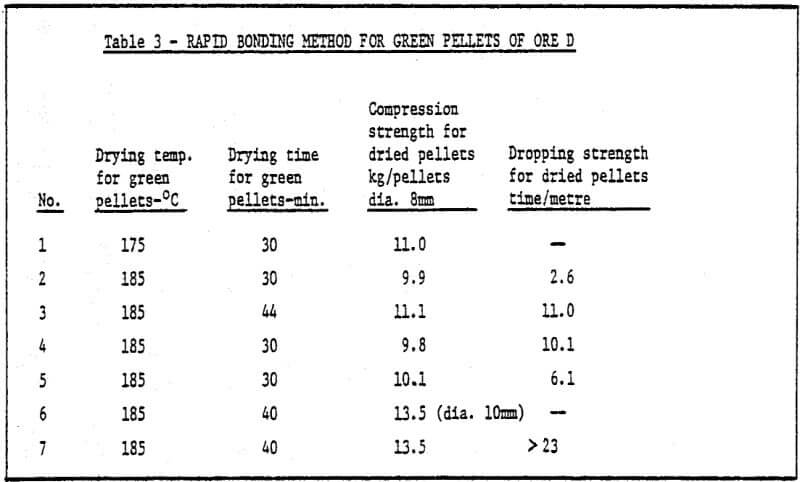
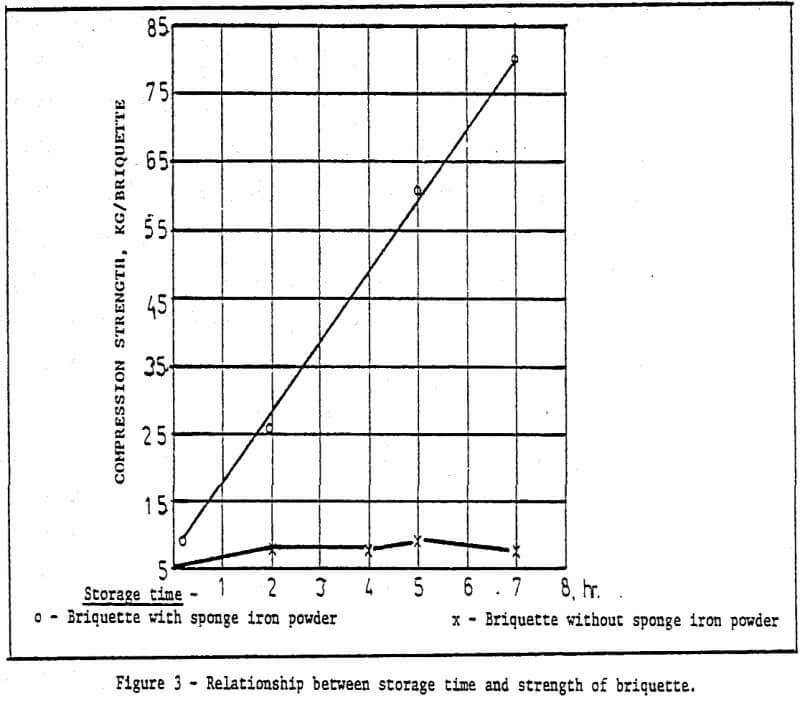
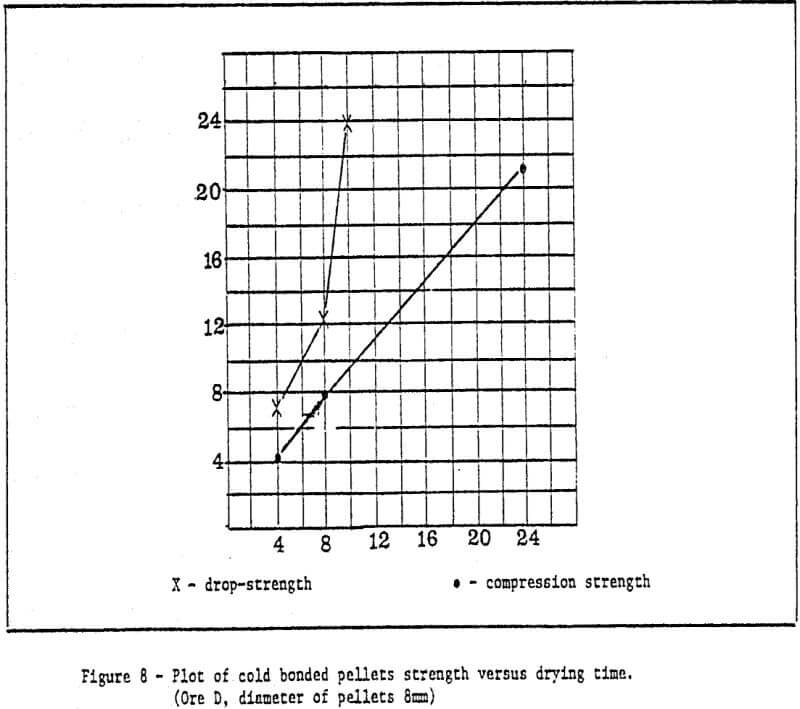
b) Method of rapid cold bonding of green pellets – Results of experiments show that only ore D is suitable for this method. Maybe the reason lies in the presence of Mn dioxide in the ore D. As a result, depolarization which promotes corrosion took place during the cold bonding process of green pellets. The rest (four) of the ores are not suitable for this method. Rapid cold bonding of ore D shown in Table 3 means producing green pellets from ore D and sponge iron powders and drying at 200°C. Thus, pellets reach desired strength.
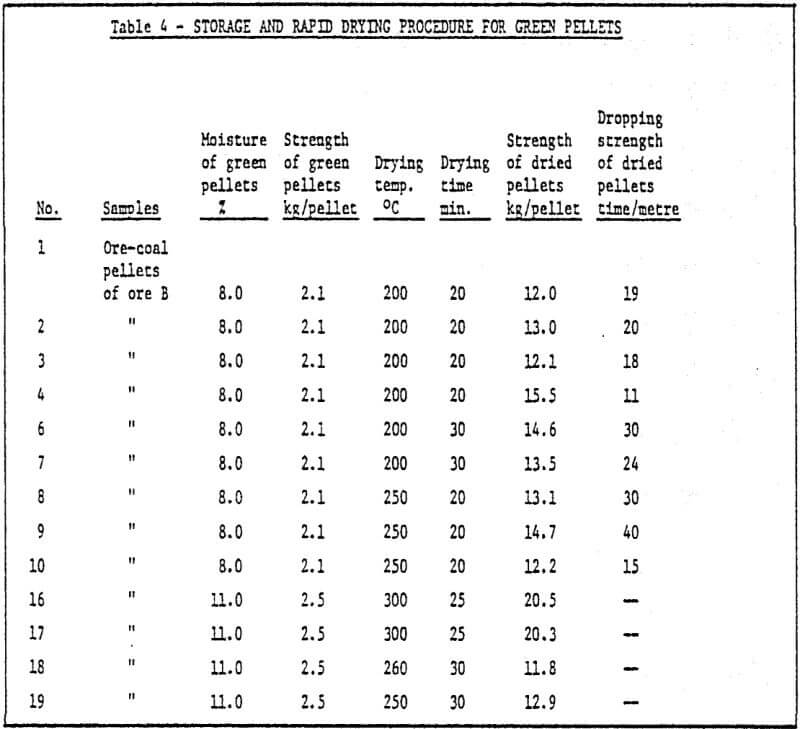
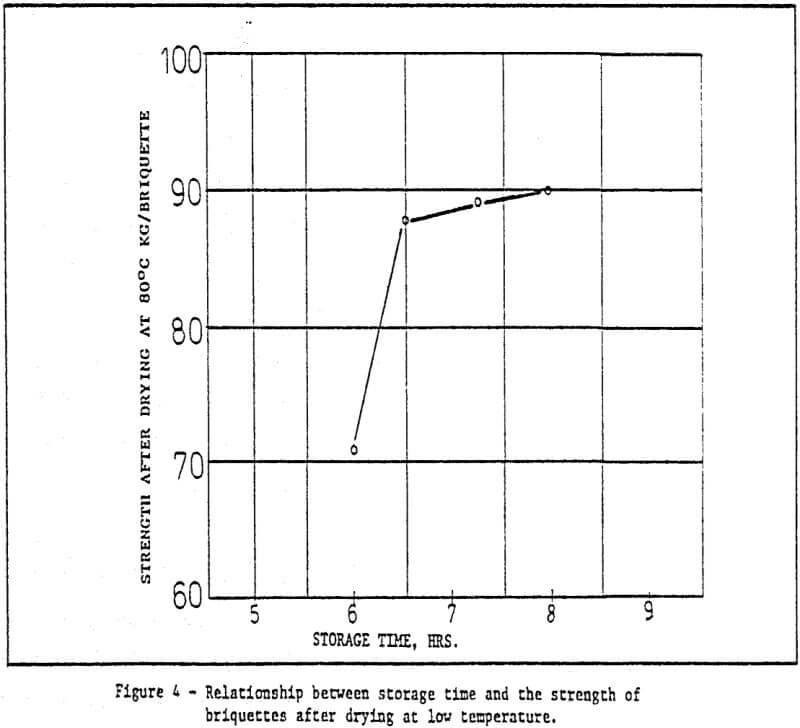
c) Storage of green pellets and rapid drying method
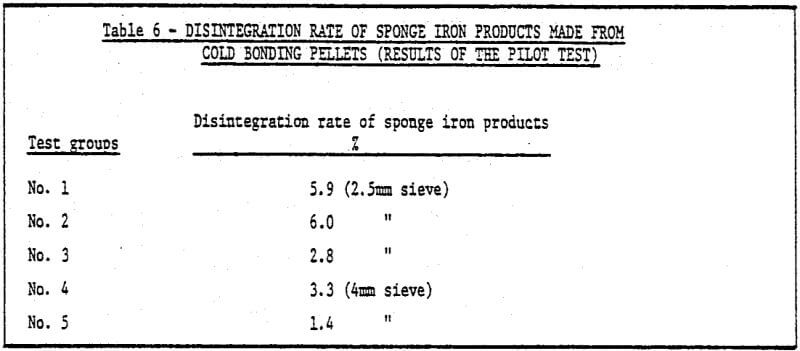
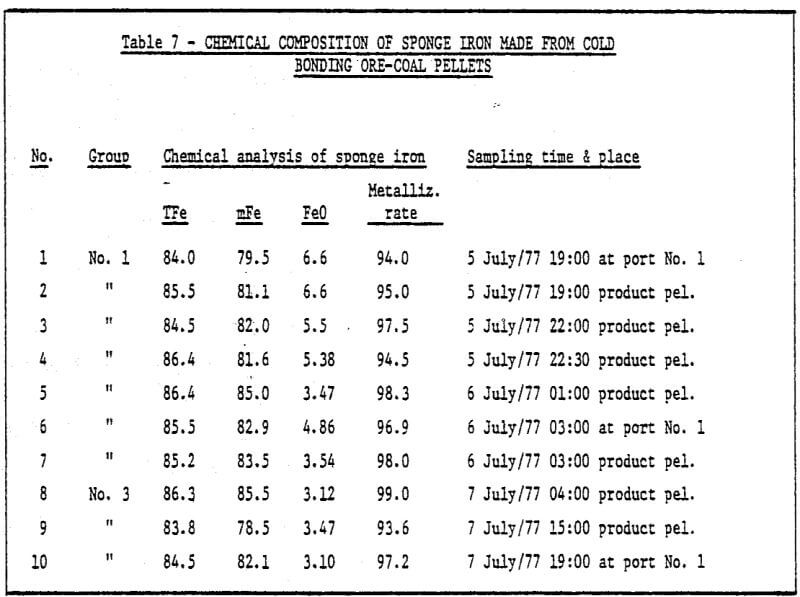
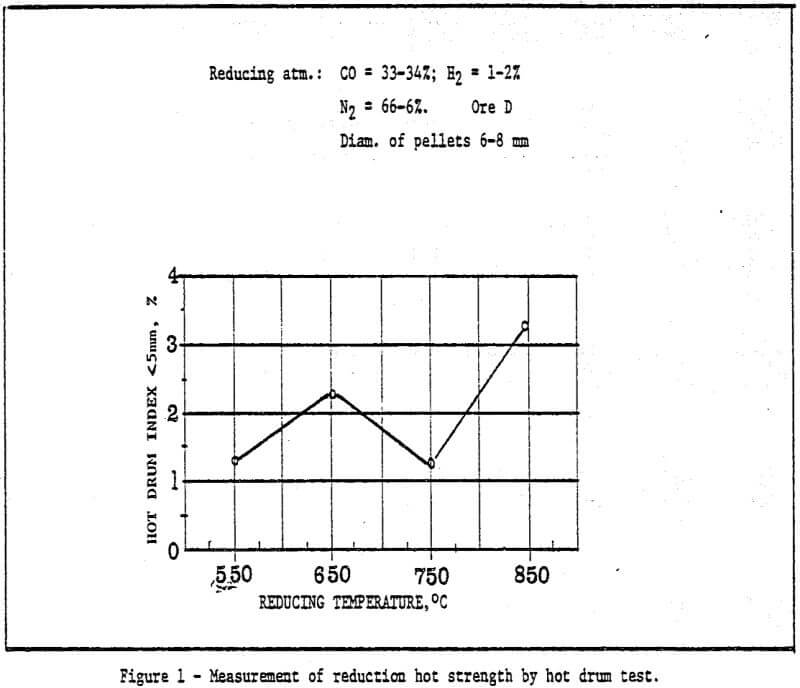
3) The study of metallurgical properties of cold bonding pellets.