Table of Contents
Granitic pegmatite deposits are the chief source of commercial feldspar, sheet mica, beryllium, tantalum-columbium, and lithium minerals, and certain types of kaolin. They also have yielded significant quantities of cassiterite, gems, scrap mica, molybdenite, tungsten minerals, uranium-thorium and rare-earth minerals, and zircon, either directly or as the sources of placer deposits. The output from pegmatite mines in the United States is small as compared to other mineral products in terms of bulk or value, and much of it comprises minor metals and nonmetals. Nevertheless, pegmatite minerals play a vital part in domestic industrial economy, particularly in the ceramic and electrical industries. Numerous special purpose uses also are important, even though they require small quantities of raw materials.
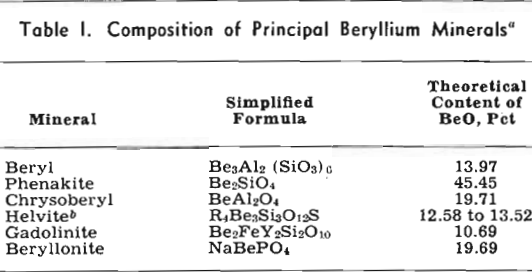
Beryllium Minerals
Beryl is the present commercial source of beryllium metal and beryllium compounds, which are used in ceramics, in the preparation of X-ray tubes and fluorescent lamps and screens, in special processes of paint and textile manufacture, and in the optical systems of some electrical instruments. The metal also is used in nuclear physics, chiefly as a source of neutrons. Beryllium is alloyed with aluminum for certain light-metal uses and is a constituent of some nickel and iron alloys. Currently the chief demand, however, is for copper-base alloys, which are exceptionally resistant to fatigue and wear, are responsive to hardening treatments after
being worked soft, and are harder and otherwise superior to copper in structural characteristics. In addition, they are good electrical conductors, non-magnetic, and nonsparking. Alloys of the beryllium-copper group are used, for example, in nonsparking tools and in springs, contact plates, bushings, shims, and corrosion-resistant parts of motors and gages. They also are employed for parts in precision instruments and machines.
Lithium Minerals
Salts of lithium are used in pharmaceuticals, storage batteries, flares and fireworks, fluxes and special lubricants, as well as in the curing of meat, manufacturing of textiles and ceramics, refining of metals, smelting of iron ore, purifying of helium,21 and dehumidifying of air in air-conditioning equipment. Lithium hydride has been employed as an effective transporter of hydrogen in self-inflating rafts, balloons, and other devices, and the metal is a minor constituent of some special-purpose alloys. The chief raw materials used for the manufacture of lithium compounds are the pegmatite mineral spodumene and the lithium-bearing brines of several saline lake deposits.
Spodumene can be employed directly in the manufacture of glass to neutralize shrinkage during cooling and also is an ingredient of some ceramic mixes. Lepidolite, the lithia mica, is much more widely used in glass making than as a source of lithium salts and is commercially as important as spodumene. Not only is it an excellent fluxing material, but it increases the luster, weather resistance, and strength characteristics of glasses, while reducing their coefficients of expansion. Such glasses are in great demand for high-pressure gages, electronic tubes, and other devices subject to mechanical stresses or sudden temperature changes. Lepidolite also is an ingredient of many high-quality porcelains and enamels in which it is an effective opacifier.
Tantalum-Columbium Minerals
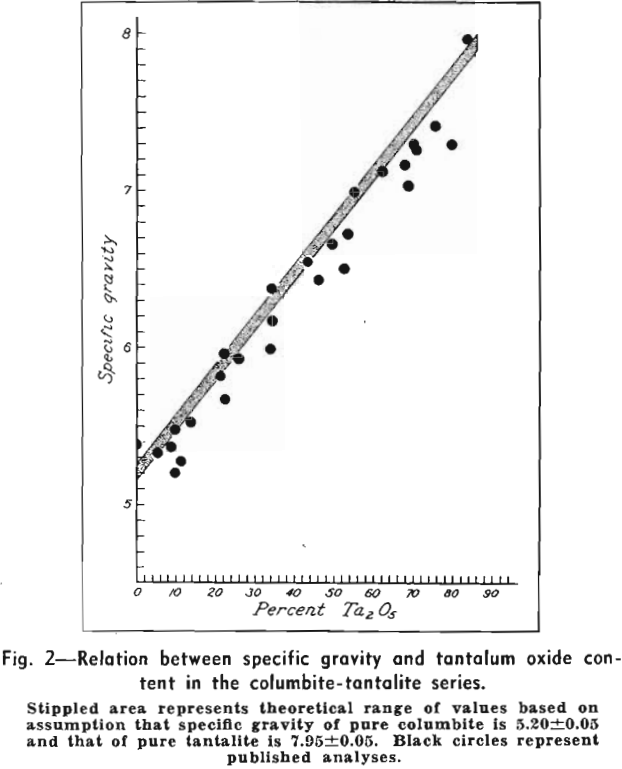
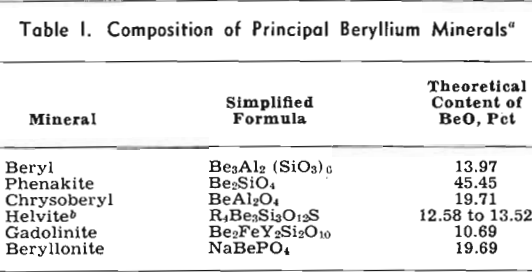
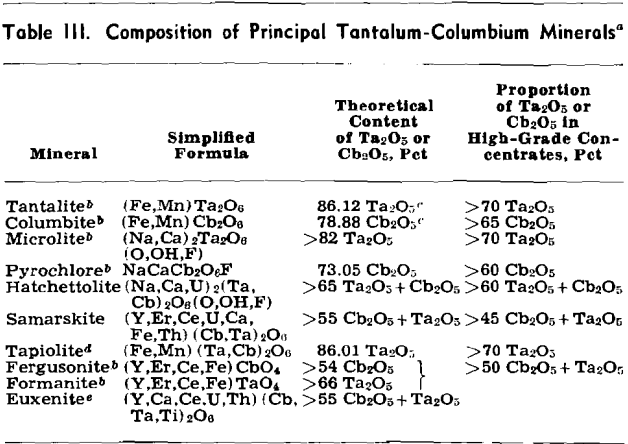
The metals tantalum and columbium are derived mainly from members of the tantalite-columbite series and to an appreciable extent from microlite and other minerals, see Table III. Columbium is alloyed with nickel, copper, and aluminum. Columbium-bearing alloy steels, with their favorable welding characteristics and high-temperature strength properties, are in demand for turbine and aircraft engine parts. Tantalum metal is used in radio and neon tubes, where its gas absorption properties are important, and its chemical inertness makes it unusually suitable for instruments and equipment that are exposed to corrosive liquids and fumes. It is employed in the manufacture of synthetic rubber and is uniquely satisfactory as a surgical metal. Tantalum is alloyed with columbium and tungsten to form dies and cutting tools, which also are made with cemented tantalum carbides. Tantalum-bearing glass is used in special camera lenses and other optical equipment.
Accurate determination of grade is accomplished by chemical analysis, a slow and costly process, but rough estimates for members of the tantalite-columbite series can be made on the basis of the consistent relation between specific gravity and Ta2O5 content.
Sheet Muscovite
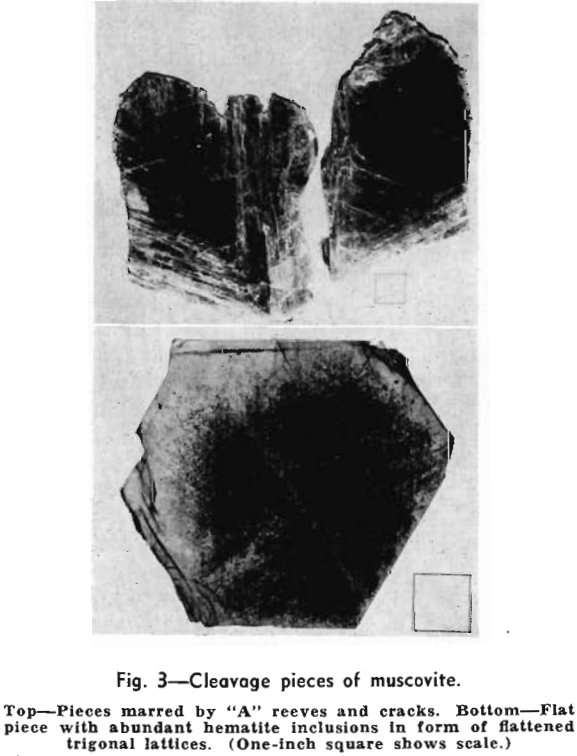
The uses of sheet muscovite are based upon its perfect cleavage, remarkably low conductivity of heat and electricity, high dielectric strength, non-inflammability, mechanical strength, flexibility, elasticity, transparency, luster, and the ease with which it can be worked into final form. The degrees of emphasis placed upon given properties by purchasers depend upon the specific end uses involved. Flexibility is particularly important, for example, in the “cigarette” mica used in spark plugs for aircraft engines. This material, in films twelve ten-thousandths of an inch or less thick, is wrapped around rodlike spindles a little more than 1/8 in. in diam. Condenser mica, in contrast, is valued because of its dielectric properties, and the use of mica for windows in furnace walls and doors is founded upon its transparency, heat resistance, and mechanical strength.
A very high proportion of sheet mica is used as an electrical insulating material. Washers, disks, and other small trimmed or stamped forms are not only employed as such, but they can be built up into
rods, tubes, or other articles by cementing them with shellac, glyptol, or a similar bonding medium. Simple and composite pieces are used, for example, as sleeves, studs, tubes, washers, bushings, laminations, and thin perforated plates in condensers, transformers, small heating elements, rheostats, fuses, incandescent bulbs, radio and electronic tubes, various types of coils, and in acoustic, X-ray, and other specialized equipment. Thin splittings are built up into mica board or applied as facing on paper, cloth, and other materials used in the manufacture of heater elements; commutators; boards, panels, and other mounting forms; parts of condensers; and many other electrical devices.
Pegmatite Deposits
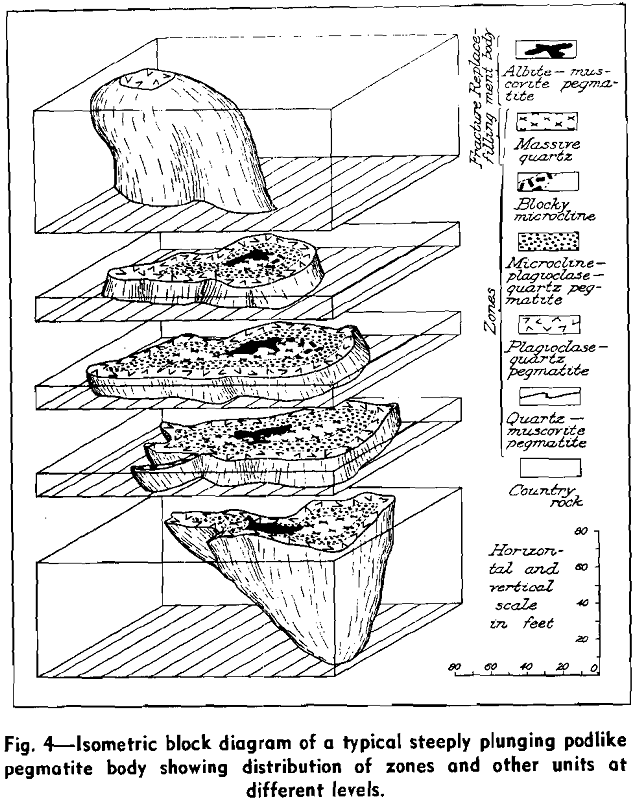
Most pegmatites can be classified as sills or dikes, depending upon whether or not they are conformable with the structure of the enclosing country rock. Variations of these forms include markedly pinching-and-swelling bodies and series of disconnected lenses and pods. In addition, troughlike, funnel-like, cigar-shaped, mushroom-shaped, and various branching forms have been recognized. Studies in all parts of the United States have shown that, despite numerous complexities of detail, most pegmatite bodies of commercial interest are rather regular in general structure. Plunging bodies are especially common, and many pegmatites that appear to be simple sills or dikes actually are shaped in three dimensions more like laths or flattened cigars, with long axes that plunge gently to moderately. Such plunging structures are extremely important as far as economic exploration of the deposits is concerned.
Pegmatite bodies that cannot be divided readily into units of contrasting composition and texture appear to constitute the great bulk of pegmatitic material in some areas. In general, however, these have received much less attention than pegmatites that are lithologically and structurally more complex. This latter group includes nearly all pegmatites that contain rare minerals, as well as most of those with minable concentrations of feldspar, mica, beryl, and other minerals. A general systematic arrangement of lithologic units in such pegmatites has long been recognized, and bands, barrels, columns, layers, lenses, pipes, pods, ribs, shoots, streaks, veins, and zones are terms commonly used by miners and referred to in geologic literature.
Although much of the tantalum and some of the beryllium and lithium minerals apparently were developed by deuteric replacement of pre-existing pegmatite, their vertical distribution is broadly controlled by the layered or zonal structure of the dike. Moreover, their along-strike and down-dip distribution is in accord with discontinuities and changes in thickness of the zones. A few small fracture-controlled replacement bodies of albite and quartz with varying quantities of beryl, microlite, tantalite, hatchettolite, and bismuth minerals transect zones and zone boundaries at distinct angles, but these bodies are not abundant and are of little economic significance.
Development of Reserves

Mining operations in pegmatite deposits have been cited repeatedly as poor financial risks, owing in large measure to uncertainties arising from the smallness and irregularity of minable concentrations of marketable minerals. Pegmatite mining traditionally has been started on promising outcrops and has been continued only until the exposed concentrations of desirable minerals were worked out or until other conditions made operations unprofitable. Thus reserves rarely are developed in advance of actual mining, and individual operations are characteristically short-lived. Moreover, the selective mining of the richest parts of mica shoots and other mineral concentrations leads to development of tortuous gopherhole workings, as well as to the leaving of much valuable material in the ground. Not only are such methods plainly wasteful, but they impose limitations on the depth to which a given shoot can be worked effectively, and they fail to uncover reserves in the form of adjacent shoots. Uncertain market conditions, limitations of available capital, and the low margin of profit commonly obtained from the sale of many pegmatite commodities militate against aggressive exploration and development of reserves. Numerous attempts have been made and a few have been conspicuously successful, but unfortunately most have antedated the accumulation of adequate structural data on the deposit being tested. In the Petaca district of New Mexico, for example, several adits and shafts were aimed at down-dip extensions of known deposits, but were developed without knowledge of the gentle to moderate plunges of those deposits. Several low-level adits that were driven to intersect productive parts of pegmatite bodies thus passed entirely beneath the keels of these bodies. Although they actually emphasize the geometric significance of the plunges involved, such disappointments nevertheless have been mentioned repeatedly in support of the thesis that pegmatite bodies are too irregular to be developed in advance of mining.
