Table of Contents
A Passive Mine Drainage Treatment Systems helps treat contaminated coal mine drainage to meet EPA and State water quality requirements.
Approach
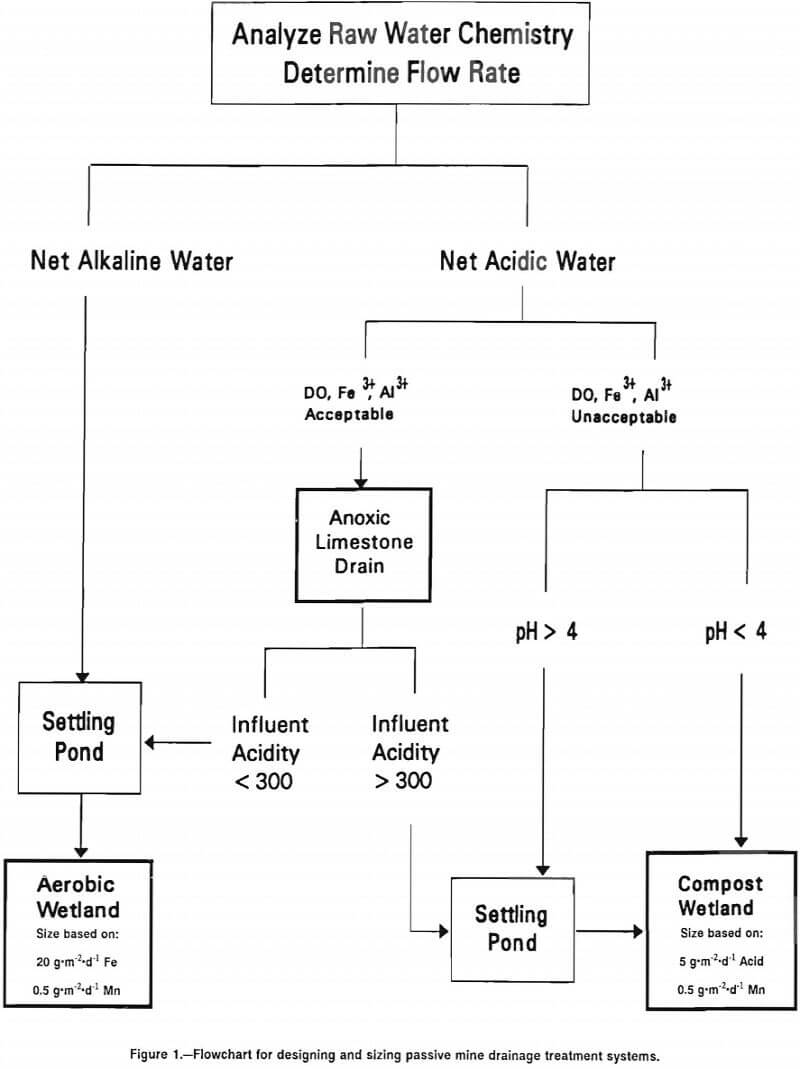
A model (fig. 1) is presented for selecting, designing, and sizing one or several passive mine drainage treatment systems that can be used in remediation of abandoned mine lands (AML). The three principal types of passive mine drainage technologies for the treatment of coal mine drainage are an aerobic system, a compost wetland, and an anoxic limestone drain.
The multistep approach presented here provides a simplified method to characterize the type and magnitude of the water quality problem and then to select either a single treatment system or a combination of treatment systems to address the specific site situation.
How a Passive Mine Drainage Treatment Systems Works
Step 1. Discharge Characterization
Characterization of the discharge consists of measuring the discharge flow rate and collecting water samples at the point of discharge for chemical analysis. Both the flow rate and chemistry of a discharge can vary seasonally and in response to storm events. It is important to account for this variability by determining flow rates and water chemistry under various conditions.
Water should be analyzed for pH, dissolved iron (Fe), alkalinity, and hot acidity (using the H2O2 method). Samples collected for metal analysis should be acidified in the field; samples containing visible particulates should be filtered before acidification.
Both acidity and alkalinity are expressed as milligrams per liter calcium carbonate (mg-L-¹ CaCO3) equivalent, so one value can simply be subtracted from the other. If an anoxic limestone drain is being considered, measurements of ferrous iron (Fe²+), dissolved aluminum (Al), and dissolved oxygen (DO) concentrations are needed. If total dissolved iron (Fe) exceeds ferrous iron (Fe²+), the difference represents ferric iron (Fe³+).
Dissolved iron (Fe) and acidity loadings (grams per day) are calculated by multiplying contaminant concentrations (milligrams per liter) by the flow rate, using appropriate conversion factors.
Step 2. Discharge Classification
The mine water is classified by comparing concentrations of acidity and alkalinity. If alkalinity exceeds acidity, the water is net alkaline. If acidity exceeds alkalinity, the water is net acid.
Step 3. Net Alkaline Discharge
Net alkaline discharge already contains sufficient alkalinity to buffer the acidity produced by metal hydrolysis reactions. Dissolved iron (Fe) will precipitate, given enough time. No organic substrate is necessary. The objective of treatment is to aerate the water and promote metal oxidation processes. An aerobic wetland, incorporating aeration features such as waterfalls or riprap ditches, will provide the necessary treatment. Experience indicates that the rate of iron removal in alkaline water is approximately 20 grams per square meter per day (g-m²-d-¹). Therefore, the size of the wetland is as follows:
Minimum wetland size (m²) = Fe loading (g d-¹)/20 g-m-²-d-¹
Step 4. Net Acidic Discharge
Treatment of acidic water requires the generation of enough alkalinity to neutralize the excess acidity. Currently, there are two passive methods for generating alkalinity: pretreatment of acidic drainage by use of an anoxic limestone drain or construction of a compost wetland.
Anoxic limestone drains (ALD’s) are essentially sealed drains designed to intercept mine water prior to contact with atmospheric oxygen. They produce alkalinity at a lower cost than do compost wetlands. However, not all water is suitable for pretreatment with ALD’s. The primary chemical factors currently believed to limit the utility of ALD’s are ferric iron (Fe³+), dissolved aluminum (Al), and dissolved oxygen (DO). When acidic water containing any Fe³+ or Al contacts limestone, both metals hydrolyze and precipitate. No oxidation is necessary for these hydrolysis reactions to occur. Ferric hydroxide can armor (surface-coat) limestone, limiting its further dissolution and benefits. The buildup of both Al and Fe precipitates within an ALD can eventually decrease the its permeability and cause plugging. While the short-term performance of ALD’s that receive water containing elevated levels of Fe³+, Al, or DO can be spectacular (with total removal of the metals), the long-term performance and the longevity of these systems are yet to be demonstrated.
In an ALD, alkalinity is produced when the mine water contacts the limestone. Using limestone with a high calcium carbonate (CaCO3) content is important because its reactivity is higher than that of a limestone with a high magnesium carbonate (MgCO3) or CaMg (CO3)2 content. The ALD must be sealed so that inputs of atmospheric oxygen are minimized and the accumulation of carbon dioxide within the drain is maximized.
The ALD should be designed so that the limestone is inundated with water at all times. This may be accomplished with clay dikes within the drain or riser pipes at the outflow of the drain. Where linear drains were not possible, anoxic limestone beds have been constructed that are 30 to 50 ft wide; they appear to work as well as the drain systems.
The ALD is only one component of this passive treatment system. When the ALD operates ideally, its only effect on mine water chemistry is to neutralize low pH and increase concentrations of bicarbonate alkalinity and calcium. Dissolved Fe will generally be unaffected by flow through the dram. The ALD must be followed by a settling basin and wetland system in which metal precipitation (i.e., Fe) reactions can occur.
Experience has demonstrated that there are three treatment situations, based on contamination level of the water. In the, first case, when DO, Fe³+, and Al concentrations are very low (<1 mg-L-¹) and net acidity is <300 mg-L-¹, the water may be treated by building an ALD linked with an aerobic wetland system. The size of the system may be determined according to the criteria provided earlier for net alkaline mine water.
The second case occurs when DO, Fe³+, and Al are <1 mg-L-¹, but net acidity is >300 mg-L-¹. Water treatment will then consist of an ALD, an aerobic wetland to remove Fe, and a compost wetland, to generate additional alkalinity.
Compost wetlands generate alkalinity through a combination of bacterial activity and limestone dissolution. The desired sulfate-reducing bacteria require a rich organic substrate in which anoxic conditions will develop. Limestone dissolution also occurs readily within this anoxic environment. Any well-composted equivalent should serve as a good bacterial substrate. Compost substrates that do not have a high calcium carbonate (CaCO3) content should be supplemented with limestone. The compost should be 12 to 18 in deep. Like aerobic wetlands, most compost wetlands are planted with cattails.
Compost wetlands in which water flows on the surface of the compost generally remove acidity (i.e., generate alkalinity) at 2 to 12 g-m²-d-¹. This range in performance is largely a result of seasonal variation, since rates of acidity removal are lower in winter than in summer. Recent studies indicate that supplementing the compost with limestone and incorporating system designs that cause most of the water to flow through the compost (as opposed to flowing on the surface) may result in higher rates of limestone dissolution and better winter performance.
A treatment system that contains all three passive technologies should be sized based on aerobic removal of Fe and removal of acidity in a compost wetland.
Minimum size (m²) = Fe loading (g·d-¹)/20 g.m-².d-¹
+ acidity loading (g.d-¹)/5 g.m-².d-¹
where the acidity loading reflects the net acidity of the ALD effluent. A preconstruction estimate of this acidity value is obtained by subtracting 300 mg-L-¹ (the estimated alkalinity generated by the drain) from the original mine water acidity.
The third case occurs when DO, Fe³+, or Al is too high for an ALD. Construction of a compost wetland is then recommended. In many wetland systems, the compost cells are preceded with a single aerobic pond in which iron (Fe?) oxidation and precipitation occur. This feature is useful where the influent to the wetland is of circumneutral pH, and rapid, significant removal of iron (Fe?) is expected as soon as the mine water is aerated. Aerobic ponds are not recommended when the water entering the wetland system has a pH below 4. At such low pH, iron (Fe) oxidation and precipitation reactions are quite slow and significant removal of iron (Fe) in the aerobic pond would not be expected. Sizing of a compost wetland is based on the following:
Minimum wetland size (m²) = acidity loading (g-d-¹)/5 g-m-²-d-¹
Studies of existing compost wetlands indicate acidity removal averages around 7 g-m-²-d-¹. To be conservative, an acidity removal rate of 5 g-m-²-d-¹ is recommended.
Benefits of Passive Mine Drainage
The prime benefit to be derived from using these treatment systems is the ability to meet water quality requirements of the National Pollution Discharge Elimination System. In addition, these treatment systems are more economical and maintenance free than conventional treatment with chemical additives.
Applications
Passive mine drainage treatment systems are applicable to any acid or alkaline mine drainage situation. The only constraint is the available area in which to place the selected system.

