Table of Contents
Pigments are used in two segments of paper making, as the major component in paper coating formulations and, in small percentages, as fillers in the paper web. Historically, the dominant use has been in coating, however, recent developments have fostered a multifaceted effort to substantially increase the pigment component in the paper furnish. Factors responsible for these developments together with their relationship to recent and forecast changes in paper making technology are reviewed. Finally, some thoughts are presented on the probable timing and extent of these changes as well as their expected impact on the future demand for the common paper pigments.
Paper Coating
The principal reason for coating paper is to improve its printability. Pigments are the main constituent of a paper coating with the various binders and additives normally comprising less than twenty percent of the total formulation. The coating provides a more uniform and receptive surface than the uncoated fiber web and, in turn, both facilitates and enhances graphic reproduction.
There are several different generic species of paper coating pigments and numerous variations within each category. The dominant material by far is kaolin, also commonly referred to as china clay. Other compositions include calcium carbonate, titanium dioxide, aluminum trihydrate, amorphous silicas and silicates, satin white (etteringite), talc, zinc oxide, and barium sulfate. The performance of a specific pigment is dependent upon its inherent properties such as chemical composition, particle shape, particle size, surface area, surface charge, brightness and whiteness, refractive index, specific gravity, reactivity, and affinity for various substances.

Just as the predicted growth in pigment demand is forecast to put increased pressure on the corresponding mineral resources, so too, the overall growth in paper volume is creating a corresponding increased demand for other paper-making raw materials. Fortunately, for the pulp and paper industry, most of its resource base is renewable. With, proper planning and timely investment adequate quantities of the various elements can be available to produce the forecast tonnage.
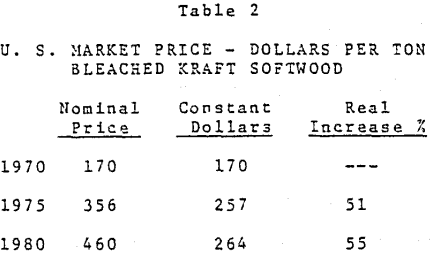
Paper formed under alkaline conditions have several inherent advantages. The first, increased permanence, has received wide attention from librarians and others concerned with the storing and maintaining of historic and artistic documents.
The second advantage of alkaline paper is directly related to its resistance to oxidation; namely, alkaline fibers lose less strength in the recycling process. This provides the opportunity for more recycling and/or the use of larger percentages of alkaline wastepaper.
With all these advantages to be gained by converting from acid to alkaline papermaking, it is hard to understand why the industry is so slow to make the conversion.
Fillers are added to paper in various percentages to perform a number of different functions. The choice of material and amount used is dependent upon the specific properties desired. While fillers can be useful in most types of paper, they are especially beneficial in printing and writing papers.
Types of Fillers
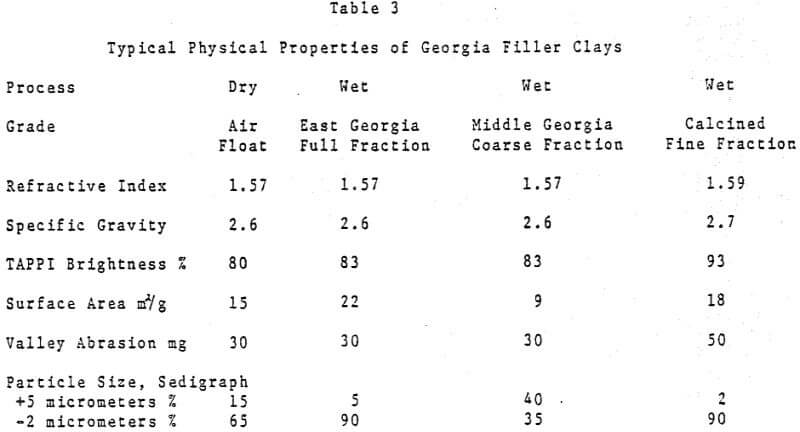
The principal fillers are kaolin clay, calcined clay, calcium carbonate, titanium dioxide, talc, aluminum trihydrate, amorphous silica and silicate. Also, a fair amount of zinc sulfide is used in Western Europe. In addition, some barium sulfate and calcium sulfate are used in special applications. Information on all of these as well as their properties, function, and consumption in paper is presented in the following paragraphs.
Kaolin Clay is the dominate material used as a paper filler with its total volume far exceeding the combined tonnage of all other filler materials. U. S. consumption as a paper filler in 1981 was estimated to be 1.4 – 1.6 million tons. Also, a very small percentage of bentonite clay is used in some paper furnishes for pitch control.
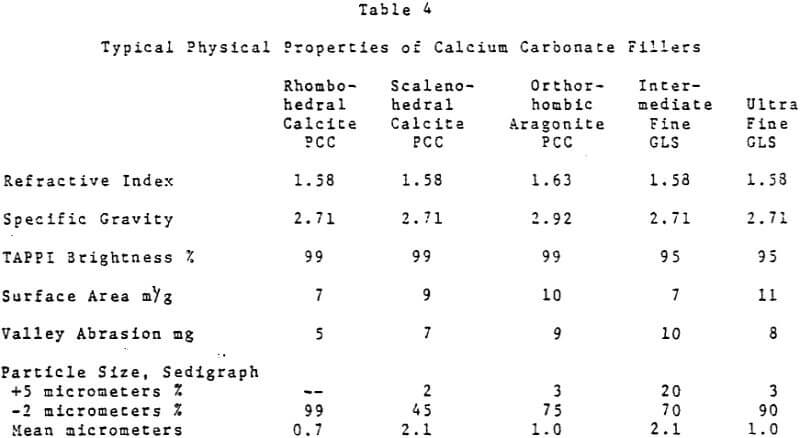
Despite its high brightness, for many years the traditional acid papermaking systems in most instances precluded the use of calcium carbonate as a paper filler. Aside from cigarette paper where the incorporation of a high percentage of precipitated calcium carbonate as a filler serves to control the paper porosity and burning rate, other uses for calcium carbonate in the paper were quite limited.
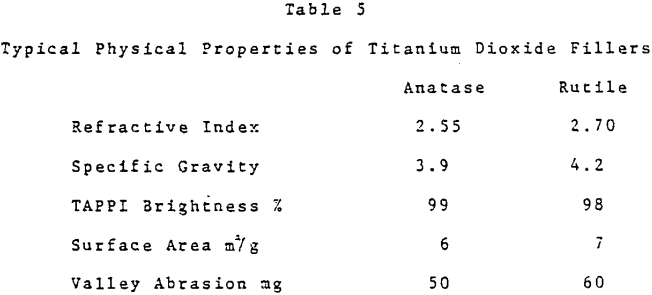
Titanium dioxide has the highest refractive index of any white pigmentary material. U. S. consumption as a paper filler in 1981 was estimated at 130,000 tons. Total titanium dioxide consumption as a paper filler worldwide was thought to be 190,000 tons.
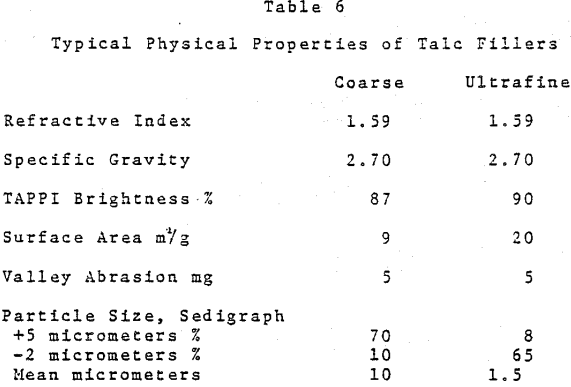
In the U. S. the major use for talc in paper is to control pitch. Because of its unique properties, there is a limited but growing interest in high purity talc as a paper filler. The situation is different in some other world areas such as Scandanavia and the Orient wherein regional availability provides economic and trade advantages as compared to imported materials.
Small percentages of synthetic amorphous siliceous pigments may be used, normally in conjunction with other paper fillers, to enhance some optical and performance properties. The siliceous pigments are characterized by an extremely fine ultimate particle that is amorphous, spherical, and generally between 0.03 and 0.3 micrometers in particle size.