Table of Contents
Take a few crystals of potassium chlorate (KClO3), place them in a clean dry test-tube, and heat them gently over a small bunsen flame; the salt begins to split, then fuses. Insert into the test-tube a splint of wood, glowing at the point, but do not allow the wood to quite touch the fused salt. The splint, which only glowed when introduced into the tube, bursts into a bright flame with a slight explosion. Withdraw the splint, blow out the flame, and again insert the glowing end into the tube ; the same result follows.
KClO3 (heated) = KCl + O3
The above equation explains what takes place in the test-tube; the potassium chlorate breaks up, potassium chloride is formed, and oxygen is evolved. This gas has the property of rekindling a glowing chip.
To prove that the potassium chlorate has undergone a change, take a crystal that has not been heated, dissolve it in distilled water, and add a drop of silver nitrate (AgNO3) solution to it. No change seemingly takes place; the solution remains clear. Now dissolve in distilled water the crystals that have been heated, and add a drop of silver nitrate solution; a white precipitate at once comes down.
KCl + AgNO3 = AgCl + KNO3.
To prepare oxygen for experiments, grind about 25 grams of KClO3 in a, porcelain mortar, remove to a porcelain dish supported on a tripod over a low bunsen flame, and keep it stirred until the salt is quite dry. Then take about 5 grams of manganese dioxide (MnO2), dry and mix well with the KClO3. Transfer the mixture to a flask fitted with a delivery tube, and set up your apparatus as shown in fig. 20 a. Fill your pneumatic trough with water, then three gas jars; invert them, and stand them on the tray in the pneumatic trough ready to collect the gas (fig. 20 b and c). Now heat up the mixture in the flask, allow time for the air in the flask to be displaced, and collect the gas by placing the jars one at a time over the hole in the tray under which the delivery tube from the flask passes.
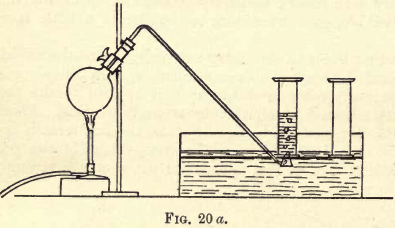
As each jar becomes full of gas slide a greased glass cover under the mouth of it, and stand it aside on the ledge of the trough ready for your experiments.
The manganese dioxide is mixed with the potassium chlorate, so that the oxygen may be given off at a lower temperature than it would if the potassium chlorate were heated alone; it does not decompose, but has simply a ‘ catalytic ’ action.
Oxygen Gas Laboratory Experiment I
Take a small piece of red phosphorus about the size of a pea, place it in the cup of a deflagrating spoon, set fire to it by bringing it into a gas flame, and plunge it into a jar of the gas (fig. 21). You will observe a brilliant white light is produced, together with dense white fumes. (Do not allow the phosphorus to burn too long in the jar, as the heat may crack the glass.) Remove the deflagrating spoon from the jar, put the cover-glass over its mouth, and allow the jar to cool. When cool, put a little water into the jar and shake it well; the white fumes will disappear, becoming dissolved in the water. Pour into the jar a few drops of blue litmus solution ; it turns red, indicating the presence of an acid.
The following equations explain the reactions:
2P + 5O = P2O5—(phosphorus pentoxide or phosphoric anhydride)
P2O5 + 3H2O = 2H3PO4—(phosphoric acid)

Oxygen Gas Laboratory Experiment II
Place a small piece of sulphur in the deflagrating spoon, set fire to it, and plunge it into another jar of the gas. You will observe it burns with a pale lavender-blue flame, far brighter than that seen when sulphur burns in air. After the flame goes out, remove the deflagrating spoon from the jar and cover the latter as before. Note the pungent odour of the substance formed, also the apparent absence of anything in the jar; the product of combustion being an invisible gas. Add a little water, shake the jar, and pour in a few drops of blue litmus solution; an acid is again indicated.
The following reactions take place:
S + 2O = SO2—(S. dioxide or sulphurous anhydride)
SO2 + H2O = H2SO3—(sulphurous acid)
Oxygen Gas Laboratory Experiment III
Place a piece of charcoal in the deflagrating spoon, set fire to it, and plunge it into another jar of the gas. You will observe a comparatively feeble light is produced. Allow the charcoal to burn as long as it will, then remove the spoon and plunge a lighted taper into the jar; it immediately goes out. Now pour in some lime-water and shake the jar; the lime-water becomes turbid, which is due to the action of the gas in the jar.
The following reactions take place:

INSTRUCTIONS TO THE STUDENT
The student has now obtained a fair knowledge of simple glass-working and of the preparation and properties of the more common gases. In the following sections he proceeds to examine solids and liquids (generally solids which are put into solution). First he examines an unknown substance by ‘dry tests’ for ‘ bases ’ and ‘ acids ’; the results so obtained are then confirmed by systematic ‘ wet tests.’
The order of work laid down is to be followed with most careful and patient attention to all details; the student has then done his part, but unless this is supplemented by equal care on the part of the demonstrator, the best results cannot be expected. The demonstrator, besides supervising the actual testing, must prepare a carefully graded series of substances leading from simple salts containing one ‘base’ and one ‘ acid ’ to complex mixtures containing four or five ‘bases’ and several ‘ acids ’ (including insolubles). The substances must all be carefully selected with the definite object of teaching the student some important point in every mixture he analyses. Indiscriminate preparation of mixtures leads to waste of time and bad work.
In his first tests the student may be given salts of known composition, and his work is then checked by unknown salts from the demonstrator’s set. For instance, on the next page he may take ZnCl2, SnCl2, Pb, Bi2S3, and so on for practice, and when fairly confident, his proficiency or otherwise is checked on ‘unknown’ salts given by the demonstrator.
The demonstrator’s ‘Record Book’ should show full details of the substances given to each student, the results obtained, the time taken with the analysis, and general remarks where necessary. A somewhat similar record must be kept of the work done in Quantitative Analysis and Assaying.
