Although a variety of inorganic and some organic reagents are used as depressants in the flotation recovery of sulfide minerals, a complete understanding of the mechanism of depressant action is still lacking. The progress in the development of depressant mechanisms have been hampered by several factors which include: tendency of sulfide minerals to oxidize in ambient conditions; inadequate control of oxygen, particularly in earlier investigations; contamination during sample preparation; surface chemical and physical heterogeneities; non-stoichiometry of many sulfides; unavailability of adequate techniques to identify surface species, etc.
Oxidation of Sulfide in the Absence of Collectors or Depressants
Pourbaix diagrams, also known as the Eh-pH diagrams, provide a comprehensive and concise representation of oxidation-reduction reactions based on thermodynamic data. Calculation of such diagrams requires a priori knowledge of the reaction products and the assumption that thermodynamic equilibrium prevails. Non-equilibrium conditions are not too uncommon for the reaction of sulfide minerals in aqueous solutions. The non-equilibrium conditions may arise because of several reasons: formation of metastable species in the solution or in the solid phase, non- stoichiometry of the solid phase, slow reaction kinetics, etc. For example, sulfur/sulfate equilibrium, which is common in oxidation of all sulfide minerals, is exceedingly slow and a large overpotential is required for sulfate to be formed at a significant rate.
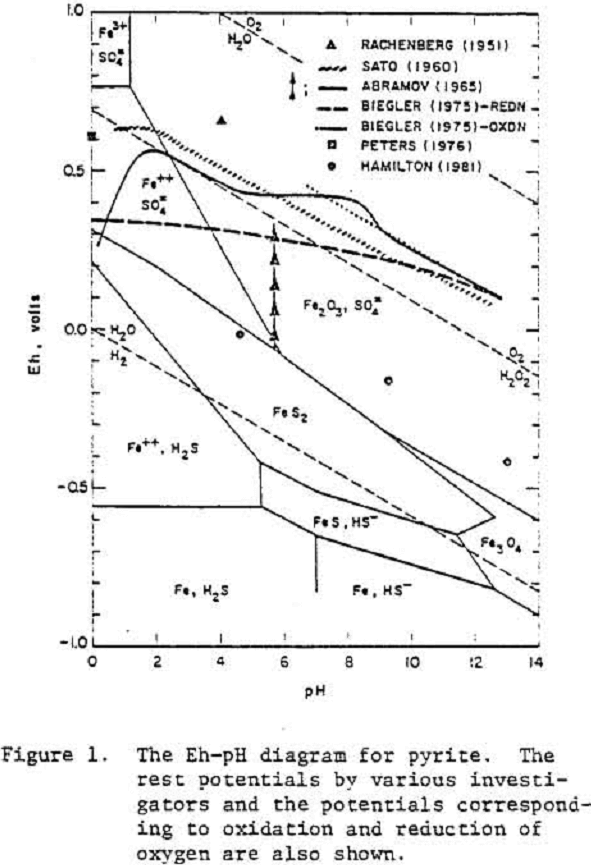
Pyrite
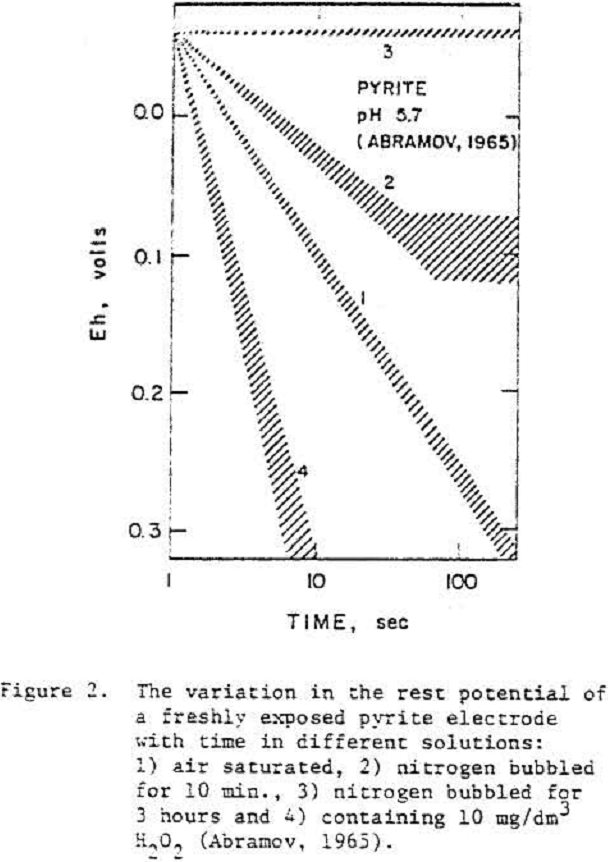
Evidently oxygen reduction occurs at a pyrite electrode which is either covered by a layer of oxide or hydroxide of iron or the electrode has a high over voltage because of the slow transformation of S° to SO4=.
The oxidation reaction of pyrite may be written as
FeS2 = Fe++ + 2 S° + 2 e-…………………………………………………….(1)
FeS2 + 8H2O = Fe+++ + 2 SO4= + 16 H+ + 15 e-………………………………………(2)
Fe++ + 2OH- = Fe(OH)2………………………………………………(3)
Fe++ + 3 H2O = Fe(OH)3 + 3H+ + e-……………………………….(4)
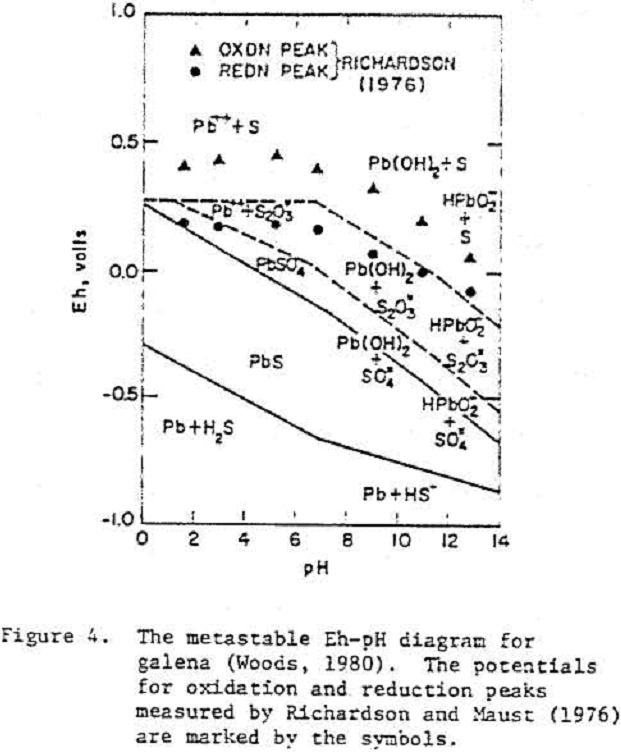
Galena
Typically, the rest potentials of freshly cleaved electrodes were close to the thermodynamic potential of galena and increased with time. The slow increase in potential after cleavage was considered to reflect an oxidation process.
PbS = Pb++ + S° + 2 e-……………………………………………..(5)
PbS = Pb²+ + (S-) + e-…………………………………………………(6)
(S-) = S° + e-………………………………………………………………..(7)
Pb²+ + OH- = PbOH+………………………………………………(6)
Pb²+ + 2OH- = Pb(OH)2………………………………………………(7)
PbS + 2 H2O = Pb(OH)2 + S° + 2H+ + 2e-………………………………………..(8)
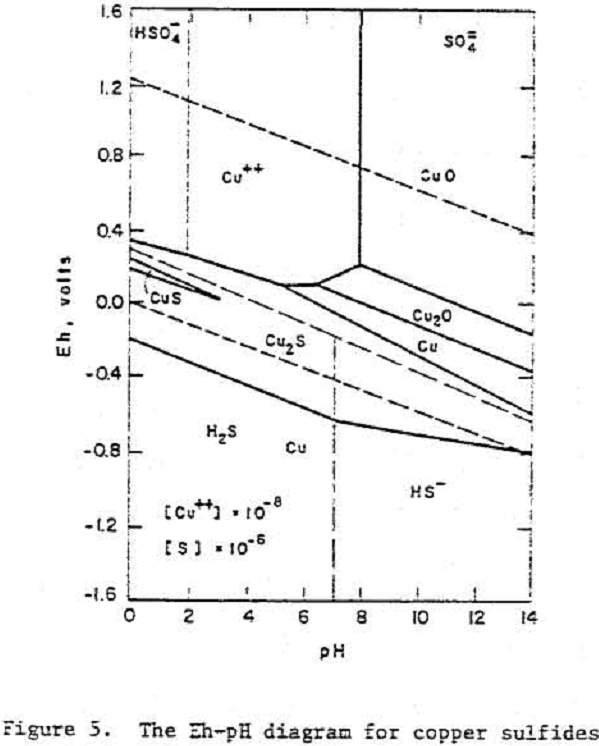
Chalcocite
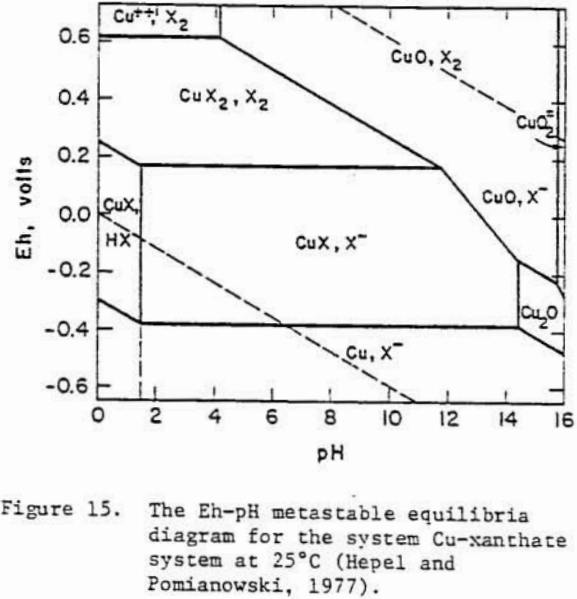
A Pourbaix diagram for copper sulfide shows that on oxidation chalcocite must decompose to give sulfate and Cu²+ ions in acidic solutions and Cu, Cu2O or CuO in alkaline solutions.
Cu2S = 2 Cu++ + S° + 4 e-………………………………………………………(7)
Cu2S = Cu++ + CuS + 2 e-………………………………………………………..(8)
CuS = Cu++ + S° + 2 e-……………………………………………………………..(9)
Cu2S + H+ + 2 e- = 2 Cu + HS-……………………………………………………..(10)
Chalcopyrite
Chalcopyrite is the cost important or the industrial copper minerals but is one of the least studied sulfide minerals, particularly with reference to its flotation chemistry.
- It is a ternary mineral, and would normally not exert a singular reversible potential in the absence of other solid phases which is in accordance with the Gibbs phase rule.
- Thermodynamically calculated Eh-pH diagrams are of little value in understanding the oxidation behavior of this mineral because its oxidative decomposition must occur with H2S (or HS-) as a reactant and FeS2 as a product. If H2S (or HS ) are not available and FeS2 cannot be nucleated, alternate Eh-pH diagrams can be drawn; one such diagram for acidic region is given by Majima (1967).
Depression of Sulfide Minerals
The various mechanisms that have been postulated include competition between depressants and collectors to adsorb at the surface sites, creation of thermodynamic conditions non-conducive for formation of a metal collector salt or formation of a hydrophilic coating on the surface.
Depression of Cooper Minerals
The effect of oxidation/reduction potentials on the flotation separation of a massive copper-nickel (chalcopyrite-pentlandite) sulfide deposit has also been investigated through measurement of Eh in the conditioning stage. The Eh of the pulp was increased by increasing the aeration rate; potentials up to 0.1 V were obtained in this way.
The effect of potential on floatability of cuprite was demonstrated at potentials greater than 0.3 V.
Depression of Pyrite
The cathodic shift in the pyrite oxidation curve for the reaction of the type
FeS2 + 2 H2O = Fe(OH)2 + 2 S° + 4 H+ + 4 e-……………………………………………(11)
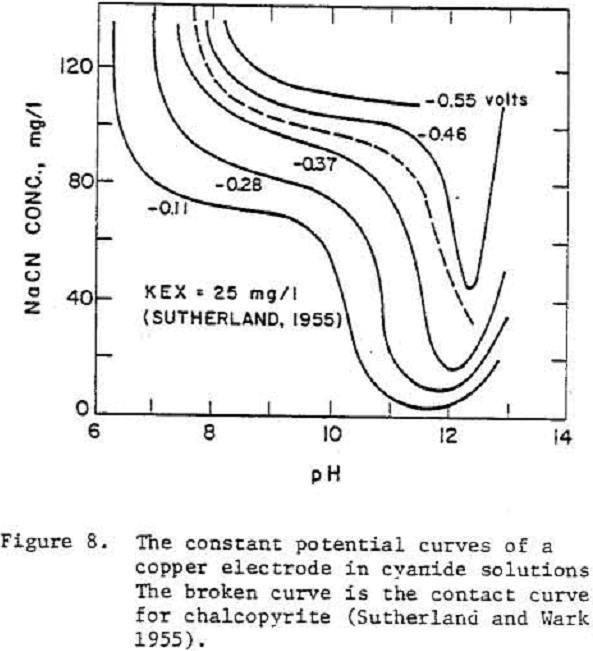
X- + 2 e- = X2……………………………………………………………………(12)
[X-]·[OH-]0.8 = constant……………………………………………………….(13)
7 Fe++ + 18 HCN = Fe4 [Fe(CN]6] 3(s) + 18 H+ + 4 e-…………………………………….(14)
The formation of solid Fe4[Fe(CN)6]3 at the surface of pyrite was considered to be responsible for the depression of pyrite. Reaction 14 is a four electron process and must involve inter-mediate steps.
log (HCN) + pH = constant………………………………………………(15)
Depression of Galena
Galena can be depressed by a variety of reagents some of which are reducing agents, for example, sulfide ions whereas others are oxidizing agents, for example, chromite, dichromate and permanganate ions.
Pb²+ + CrO4= = PbCrO4……………………………………………………………….(16)
Pb(OH)2 + CrO4= = PbCrO4 + 2 OH-…………………………………………….(17)
Even though only a limited information is available regarding the electrochemistry of depressant action of galena, most of the postulated mechanisms involve electrochemical reactions unless galena is heavily preoxidized.
PbX2 + S2O3= = PbS2O3 +2X-……………………………………………..(18)
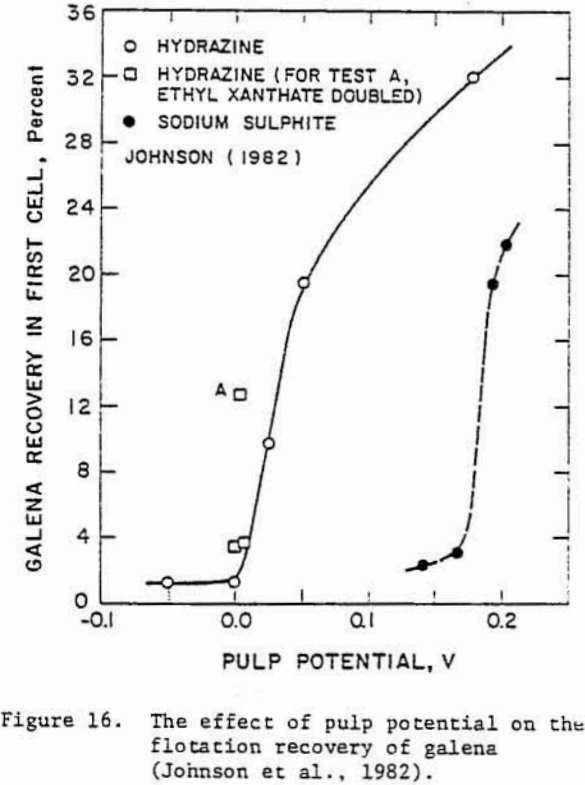
whereas the depression of galena in hydrazine may be due to the decomposition of lead xanthate at reducing potentials according to the reaction
PbX2 + (S) + 2 e- = PbS + 2 X-…………………………………………………….(19)
Summary Discussion
Sulfide minerals may be grouped into two types: reversible and passivated, based upon their electrochemical behavior in aqueous solutions. The reversible group of sulfide minerals include galena and chalcocite. The potentials for oxidation/reduction reactions for reversible sulfides can be predicted if metastabillty of elemental sulfur is considered. In the passivated group of sulfide minerals, which may include pyrite, chalcopyrite and bornite, the reactions are irreversible. The surfaces of passivated sulfides are normally covered with a layer of oxidation products. The potential of such surfaces cannot be thermodynamically predicted but are “mixed potentials”.