Table of Contents
Cement copper production has been increasing steadily because more mine waste from open pit mining is being leached and the copper recovered by cementation on scrap iron. Current estimates indicate that about 13.1 percent of the Nation’s production of primary copper is from this source. Cement copper is usually a crude product containing elemental copper, copper and iron oxides, and impurities derived from the scrap iron. Most cement copper is smelted with copper sulfide flotation concentrates in a reverberatory furnace and, consequently, a product containing as much as 90 percent copper is refined along with material containing about 27 percent copper. Alternate refining methods are needed to more efficiently utilize this source of primary copper.
The Federal Bureau of Mines has investigated electric smelting of cement copper as a possible processing route. The research showed that a product, containing 99.8 percent copper, could be obtained by induction or arc-furnace smelting with a reducing agent and flux. Refining the molten copper by oxidation and gaseous reduction or by chlorine lancing resulted in a marketable metal containing 99.9 percent copper. The Bureau of Mines has also investigated the use of mineral beneficiation techniques to upgrade cement copper. The results showed that a process comprising screening, flotation, chemical digestion, reduction, sintering, and grinding would yield a powder product containing about 98.8 percent copper and having physical characteristics suitable for powder metallurgical applications.
Currently, a small quantity of cement copper is processed by hydrometallurgical techniques in which the copper is dissolved in hot, aerated H2SO4 solutions and subsequently recovered as a high-grade product. Bagdad Copper Corp recovers redissolved copper by hydrogen reduction at elevated temperature and pressure to produce a molding-grade powder. Other operators recover the copper by electrowinning.
The currently reported investigation is the result of continuing interest in new processing routes for cement copper and is primarily concerned with oxidation roasting and acid leaching as an alternative to leaching with hot, aerated H2SO4.
Initial oxidation roasting experiments were made with cement copper in a static bed under a draft of air. The results showed that an inordinately long roasting time was required for complete oxidation. For example, when the furnace temperature was 355° C and the copper depth was 11 millimeters, the required time was 2 hours. Increasing the furnace temperature resulted in a hard sinter cake and did not oxidize the copper at a faster rate. In addition, the oxidation reaction was exothermic, and the bed temperature was as much as 100° C higher than the furnace temperature. Ellwood and Weddle experienced similar results when they attempted to oxidize scrap copper wire in bulk. The temperature increase experienced with bulk oxidation, and thermodynamic calculations, indicated that flash oxidation of finely divided cement copper might result in a more reasonable roasting time. Therefore, this investigation was confined to flash roasting to produce an acid-soluble oxide.
Most copper oxidation studies have been made on sheets and wire to obtain information on oxide film and scale formation. Copper oxide films on copper have been described as the layered series–Cu, Cu2O, CuO. Experimental studies of the oxide film indicate that the CuO content of the film ranges from a relatively high value at 400° C to zero at the decomposition temperature of CuO (1,053° C in air). Although the composition of the oxide at temperatures below about 400° C has not been well established, Tylecote reported that the CuO content undergoes a minimum between 400° C and room temperature.
Bridges studied the oxidation of copper to Cu2O and CuO between the temperatures of 600° and 1,000° C at oxygen pressures from 0.026 to 20.4 atmospheres. The oxidation data was found to fit a modified parabolic rate law above 700° C and a simple parabolic law at 600° and 700° C. The activation energy for the process was found to be 37,000 calories per mole. Inasmuch as the activation energy for the diffusion of Cu ions through solid Cu2O has been found to be 37,000 calories per mole, the rate controlling process was attributed to diffusion of Cu+¹ ions through the layer of Cu2O.
Reviews of copper oxidation have been presented by Tylecote, Ronnquist and Fischmeister, and Kubaschewski and Hopkins. In general, logarithmic and inverse logarithmic rate laws govern the low-temperature oxidation of copper, parabolic and cubic laws control copper oxidation at intermediate temperature up to about 700° C, and parabolic laws govern the oxidation at higher temperatures.
Ronnquist and Fischmeister indicated that formation of cuprite by the reaction
2Cu + ½ O2 ↔ Cu2O………………………………………………….(1)
follows a modified parabolic rate law while the oxidation of Cu2O to CuO by the reaction
Cu2O + ½ O2 ↔ 2CuO………………………………………………(2)
is governed by a purely cubic rate law.
Materials and Procedure
The cement copper used in this study was upgraded by flotation to reject iron and insoluble gangue material. The flotation concentrate was filtered, washed, dried at 100° C, and ground to pass 325 mesh. The average particle diameter was determined microscopically to be 5.2 microns. Chemical composition, in percent, was 94.5 copper, 0.45 iron, and 0.15 insoluble material. An acid leach of the dried cement copper showed that 16.4 percent of the copper was oxidized and soluble.
The flash roaster used in this first part of the study was a 1-inch-ID, vertical tube 5 feet in length heated by an electric furnace. The temperature profile in the first 12 inches of the reaction zone varied about ±25° C, and the profile in the remaining 24 inches of the reaction zone varied only about ±5° C. This roaster was used to obtain preliminary copper oxidation results and to establish design criteria for a longer furnace. The roaster used for the latter part of this study was a 1-inch-ID, vertical tube 9 feet in length heated by an electric furnace. The increased length of this furnace provided a significant increase in retention time. The furnace temperature profile varied about ±25° C over the length of the 92-inch reaction zone. Figure 1 is a drawing of the flash roasting system.
Transporting gas flow and composition was controlled by pressure regulators, valves, and flowmeters connected to compressed gas sources. Cement copper was carried by the fluidizing gas into the bottom of the furnace so that the copper was blown up through the furnace, and then recovered in a settling system at the top of the furnace.
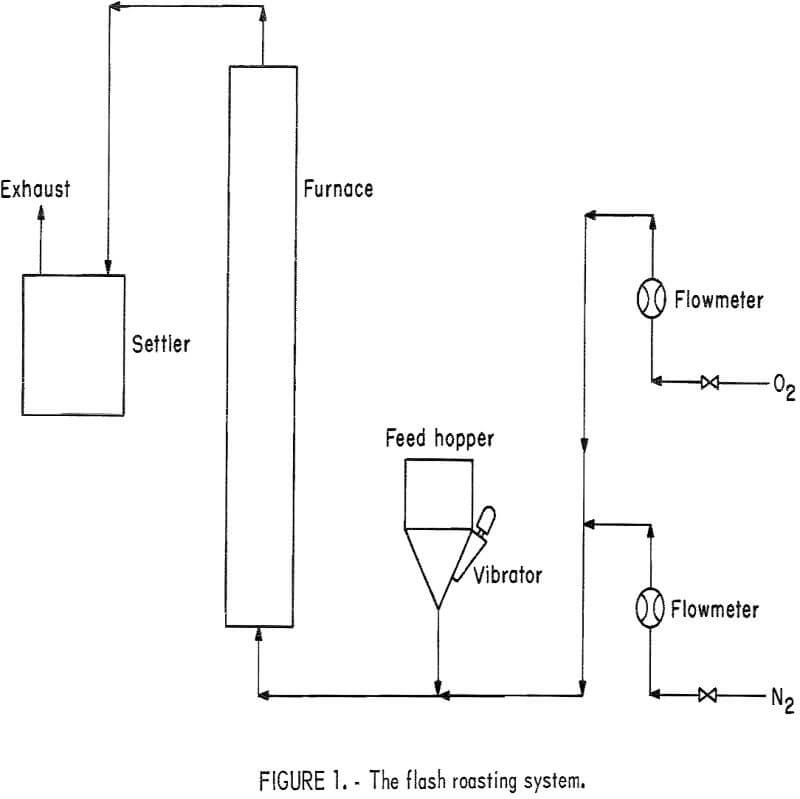
The roasting procedure consisted of feeding 50 grams of cement copper to the heated roaster at a rate of about 3 grams per minute. The total gas flow was 8,800 cubic centimeters per minute, measured at room temperature and pressure (RIP). The ambient temperature and pressure were, respectively, 25° C and 645 millimeters of mercury. With these conditions, a transporting gas containing 20 percent oxygen and 80 percent nitrogen will provide 3.3 times the theoretical oxygen requirements. Variations in retention times were attained by maintaining the gas composition constant and varying the total gas flow.
The effectiveness of the oxidation roast or the percent conversion to acid-soluble copper oxide was determined by leaching 5.0 grams of the roaster product in 100 milliliters of 11-percent sulfuric acid solution for 2 hours. During the 2-hour leach, about 5 percent of the total extraction was due to air oxidation in the leach solution. Since the same amount of air oxidation occurs in each leach, no correction was made for this anomaly.
Oxidation Roasting Results
Roasting tests were made in both the 5-foot and 9-foot furnace to determine the effects of (1) gas composition, (2) roasting temperature, and (3) retention time. The main difference in operation of the two furnaces was the retention time. With a gas flow of 8,800 cubic centimeters per minute, RTF, and a roasting temperature of 400° C, the 5-foot furnace provided a retention time of 1.71 seconds, whereas the time in the 9-foot furnace was 3.57 seconds. With increased roasting temperatures, the roasting times were correspondingly shortened due to gas expansion and increased velocity in the hot zone of the furnace.
The amount of slip between the gas and the particles was estimated from the force balance on a particle using the Stokes relation for the friction force. For a spherical particle under conditions yielding a Reynolds number less than 1.0, the velocity difference between the particle and the gas is given by
v = D²g(ps – p)/18µ………………………………………………..(3)
where v is the velocity difference, D is the particle diameter, g is the acceleration of gravity, pg is the particle density, p is the gas density, and M is the gas viscosity. The maximum percentage difference between velocities was 2.2 percent, and consequently, the particle and gas velocities were considered to be the same.
Oxygen Concentration Effect
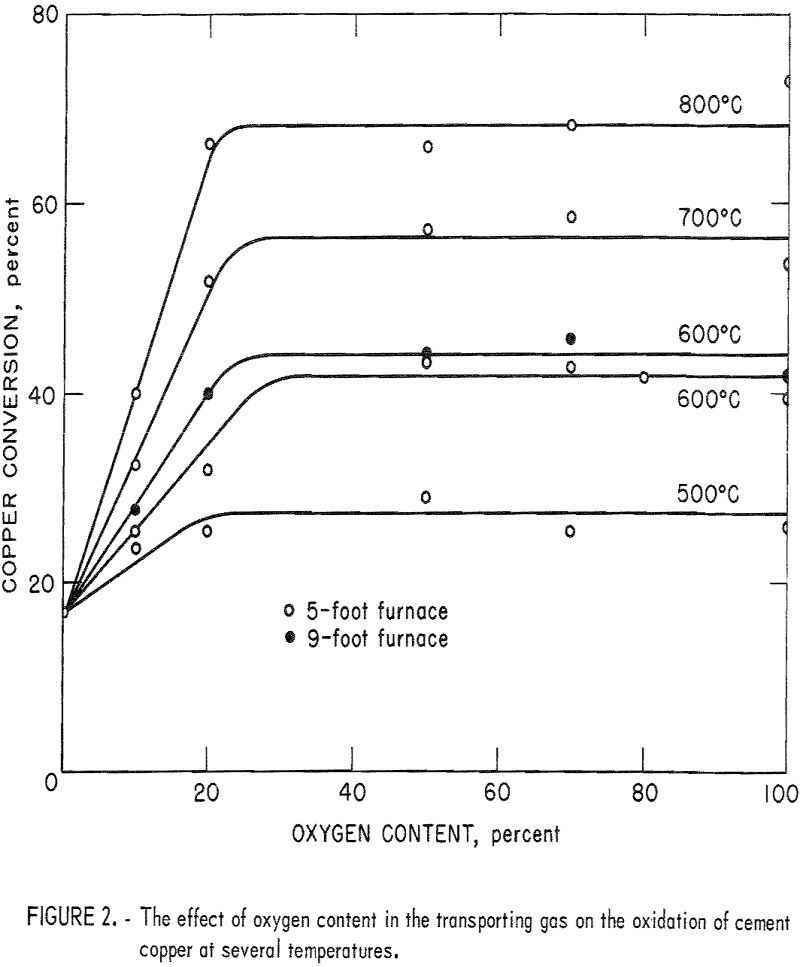
The first series of experiments was made to determine the effect of oxygen content of the transporting gas at various roasting temperatures. The 5-foot furnace was used for most of these tests; however, one series at a roasting temperature of 600° C was completed in the 9-foot furnace. The test results are presented in figure 2, which shows that the oxidation increases linearly as the oxygen content increases from 0 to 20 percent. When the oxygen concentration was above 20 percent, the extent of cement copper oxidation was constant. Therefore, a reaction gas containing about 20 percent oxygen (air) will yield maximum conversion to copper oxide.
Comparison of the results from the 5-foot furnace and the 9-foot furnace with a roasting temperature of 600° C shows that the additional roasting time attained with the taller furnace increased the maximum copper conversion by about 2.5 percent. Additional tests in the 9-foot furnace with a 50-percent-oxygen transporting gas showed that maximum conversions were increased similarly at roasting temperatures of 700° and 800° C. Thus, major increases in roasting time and/or oxygen content of the roasting atmosphere did not result in a corresponding major increase in copper oxidation. Apparently, as the copper particle moves through the roaster, the surface becomes oxidized and the oxide layer inhibits further oxidation. Also, reactions may occur to establish an equilibrium between Cu, Cu2O, and CuO.
Temperature Effect
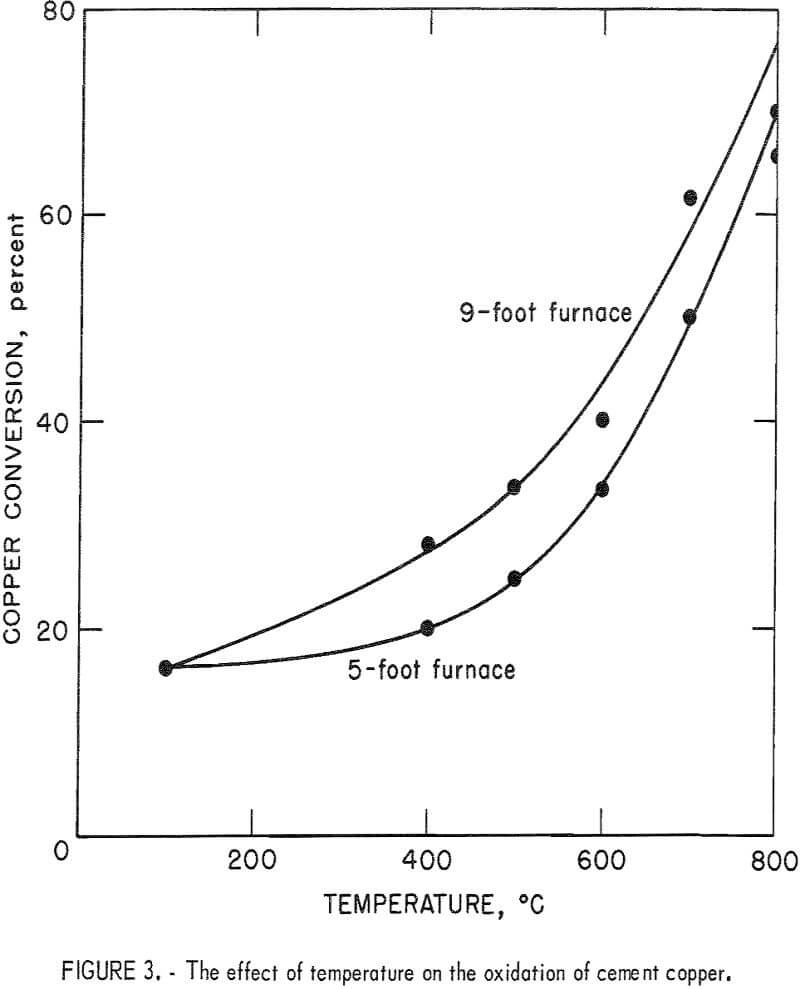
Another series of experiments was performed to determine the effect of temperature on the copper oxidation rate. A synthetic transporting gas
initially containing 20 percent O2 was used in all tests of the series. Some duplicate tests were made with air yielding similar results. Figure 3 illustrates the results of this study and clearly shows the marked increase in oxidation rate with increased temperature. Also, higher conversions were attained with the 9-foot furnace as compared with the 5-foot furnace because of the longer retention time achieved with the larger furnace. Arrhenius plots of the data in figure 3 gave activation energies of 10.17 and 10.12 kilo-calories per mole for the 5- and 9-foot furnaces, respectively. Since these activation energies do not correspond to 37 kilocalories per mole as calculated by Bridges, the rate controlling step is apparently not diffusion of Cu+¹ ions through a Cu2O layer.
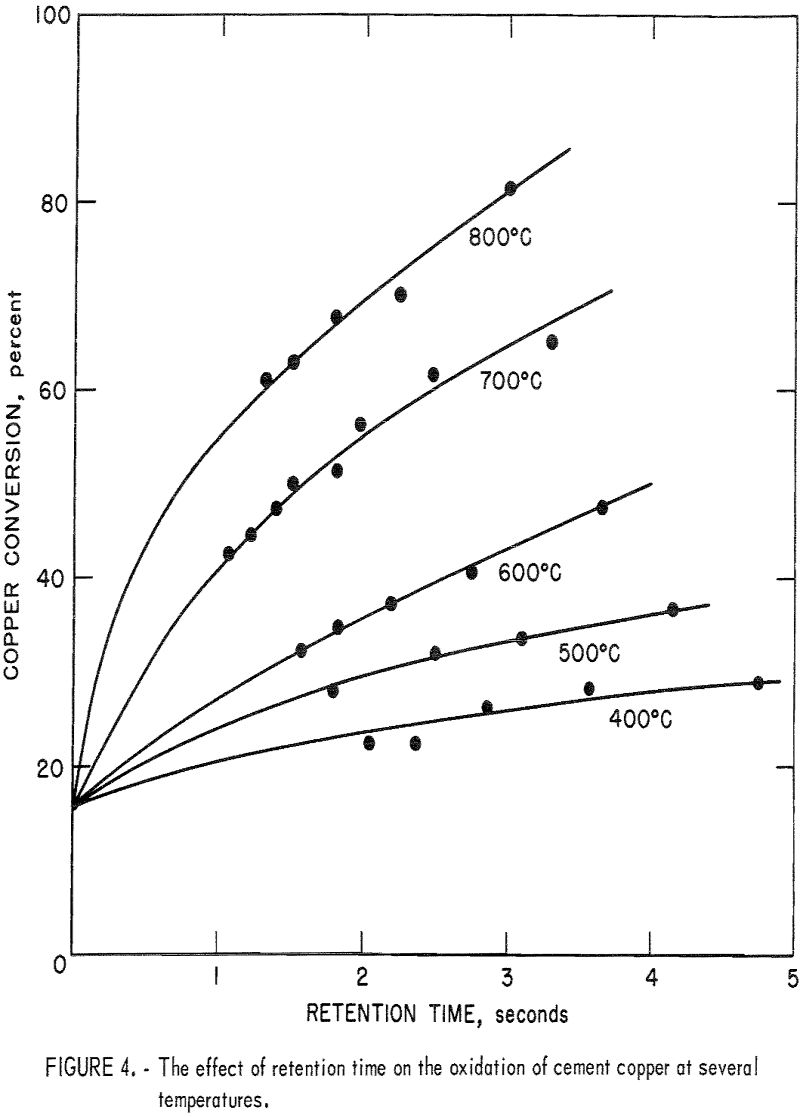
Retention Time Effect
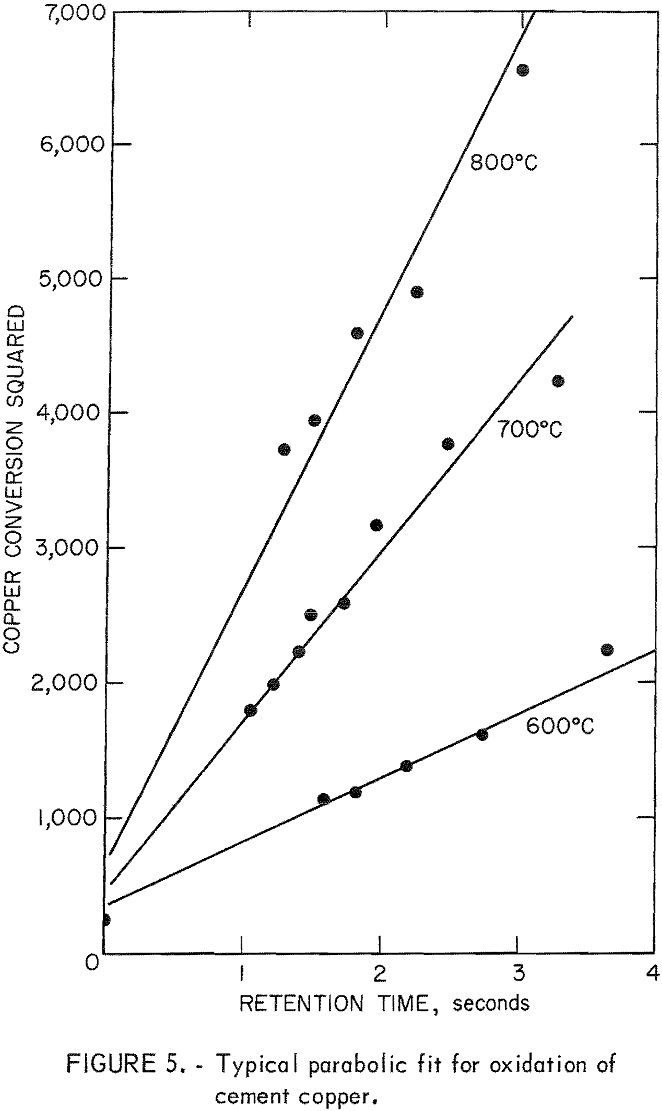
Experiments were performed to determine the effect of retention time on the oxidation reaction and to establish an empirical oxidation equation. The retention time was varied by changing the gas flow to the furnace while holding the input gas composition constant at 20 percent oxygen. Figure 4 illustrates the oxidation results at 400° to 800° C in the 9-foot furnace. The furnaces were not designed for high temperature and, therefore, 800° C was the maximum temperature that could be safely attained in this study. Figure 5 is a plot of percent extraction squared versus retention time, which shows that the oxidation reaction follows a parabolic law of the form
(E)² = mt + b………………………………………………………….(4)
where E is the percent oxidation, and m and b are, respectively, the slope and y-axis intercept of a plot of (E)² versus t.
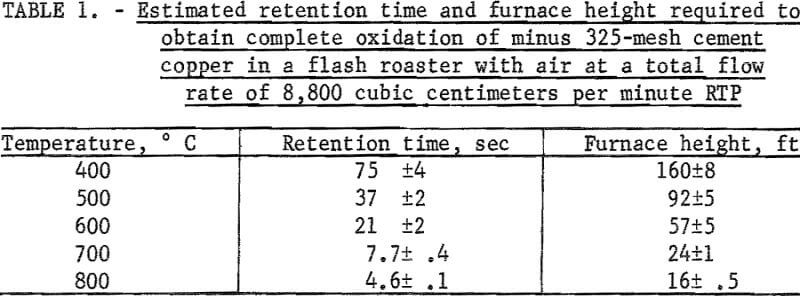
The retention time required to obtain complete oxidation can be estimated by assuming that the oxidation reaction follows the parabolic equation. The retention time can be used to estimate the height of furnace required to obtain complete oxidation, assuming that a longer furnace reacts in a manner similar to the furnaces used in this study. Table 1 lists the estimated retention times and furnace heights required for complete oxidation at a gas velocity of 30 centimeters per second RTP, which was experimentally determined to be the minimum gas velocity capable of transporting the copper particles.
Cyclic Roasting
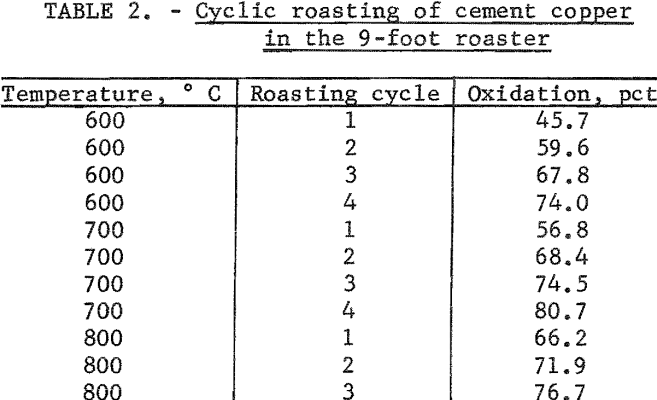
Cyclic roasting tests were performed at temperatures of 600°, 700°, and 800° C by returning the oxidized product to the roaster several times. This procedure further lengthened the total retention time in the furnace. Air was used as the transporting gas throughout the entire test. The results, which are listed in table 2, do not conform to the parabolic curves obtained by varying the gas flow rate and thus cannot be used to verify the parabolic extrapolations. Several factors probably contribute to cause the difference in results. First, the retention time is not precisely a multiple of the single-cycle retention time owing to the uncertainty in the time required to bring the unreacted core of the recycled particles up to reaction temperature. Second, the reactions that occur during recycle may not be the same as those that occur in the initial cycle. In addition, fines were lost during the first cycle causing the average particle size to increase.
Conclusions
- The oxidation of cement copper in a flash roaster is a linear function of oxygen concentration between 0 and 20 percent oxygen. There is no further increase in oxidation at oxygen contents above 20 percent, and therefore, air is an optimum oxidizing gas in this system.
- The oxidation rate increases as the temperature is increased from 400° to 800° C. The activation energy for the oxidation process is 10.1 kilo- calories per mole.
- The oxidation reaction follows a parabolic law between 400° and 800° C. Extrapolation of the parabolic law at 400°, 500°, 600°, 700°, and 800° C indicates that the retention times for complete oxidation are 75, 37, 21, 7.7, and 4.6 seconds, respectively.
- Cyclic roasting of cement copper at 600° and 700° C produced 74 and 81 percent oxidation, respectively, after four roasting cycles. Three roasting cycles at 800° C produced 77 percent oxidation. These tests could not be used to confirm extrapolation curves for the retention time in the furnace.
