The current meaning of the term, copper overpoled in the reverberatory furnace, is that poling has been carried beyond the tough-pitch stage, with the result that the reduction has been carried too far, causing the copper to become porous and brittle, and thus unfit for industrial purposes. It will be shown that the brittleness of furnace-overpoled electrolytic copper must generally be attributed to other causes than over-reduction. The present investigation, dealing with such pure metal as electrolytic copper, excluded the consideration of the effects that elements like arsenic, antimony, lead, bismuth, nickel, etc., might have if present in the oxidized or the metallic state; it confined itself to the remaining active agents, cuprous oxide, gases and temperatures, and incidentally to sulphur and iron. The plan of work was to examine samples of tough-pitch and furnace-overpoled copper from the same charges as obtained from works, to eliminate all the oxygen from the tough-pitch copper by reduction in a crucible, and to compare the results.
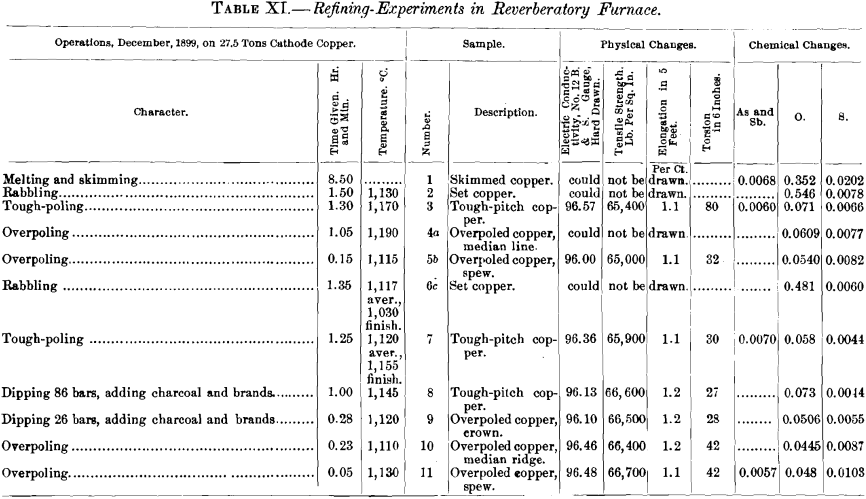
(a) Samples.—In addition to the samples from the two refining-charges (Nos. VIII. and IX., Table IX.) discussed in the first part of this paper, there were examined five specimens of electrolytic copper from Eastern works (samples Nos. I., III., IV., V., VI.), one of casting-copper (sample No. VII.), and one of native copper (sample No. X.). The samples (Table IX.) are marked with Roman numerals; the letter A affixed to a numeral designates the sample as tough-pitch copper furnace-overpoled at the works, the letter B as tough-pitch copper crucible-overpoled in the experiments. The results are assembled in Tables IX and X. Further data in regard to furnace- overpoling are given in Table XI., in which have been brought together some facts of an experimental run made by a Western plant in 1899 with a charge of 27.5 tons of cathode copper. The charge was brought to the tough-pitch stage in the usual way, overpoled, rabbled again to convert the overpoled copper into set copper, poled to tough-pitch copper, and again overpoled.
(b) Crucible-Overpoling.—The object of overpoling in a crucible was to eliminate by means of charcoal and by the exclusion of air all the oxygen of a sample and thus obtain what may be termed true overpoled copper. The apparatus used is shown in Fig. 22. The reducing crucible, A, was made of Acheson graphite, which is practically free from impurities. The cavity, 13/16 in. in diameter and 3.5 in. deep, was bored into a stick 1.5 in. in diameter and 4 in. long. The graphite crucible was placed in a size G fire-clay crucible, B, packed with crushed firebrick, C, the tops of the graphite crucible and the packing were covered with a layer of charcoal, D, 0.5 to 0.75 in. deep, and the clay crucible closed with a lid. Filings and chippings from
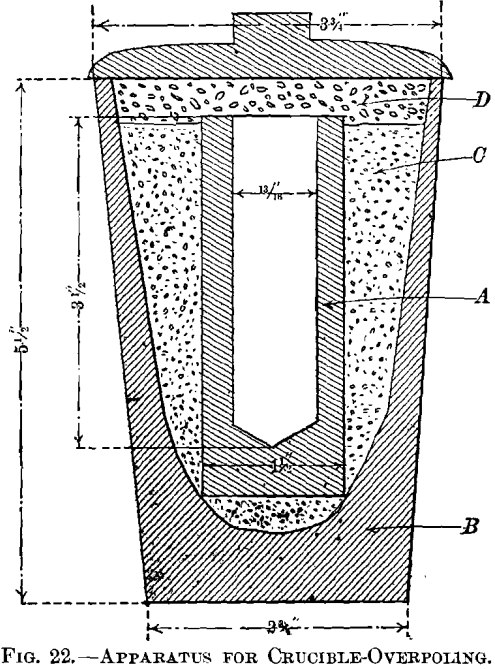
tough-pitch copper were charged, and the apparatus then placed in a pot-furnace. When the charges had been fused, more copper was added to about fill the graphite crucible, the layer of charcoal spread over it, the copper kept molten about 15 min., the apparatus removed from the furnace, and allowed to cool slowly with a layer of charcoal still on top of the copper. When cold, the copper cylinder could be easily removed. A graphite crucible was found to stand four or five heatings without cracking. As air was not wholly excluded at first during the melting-down of the copper, the upper edge of the graphite crucible was
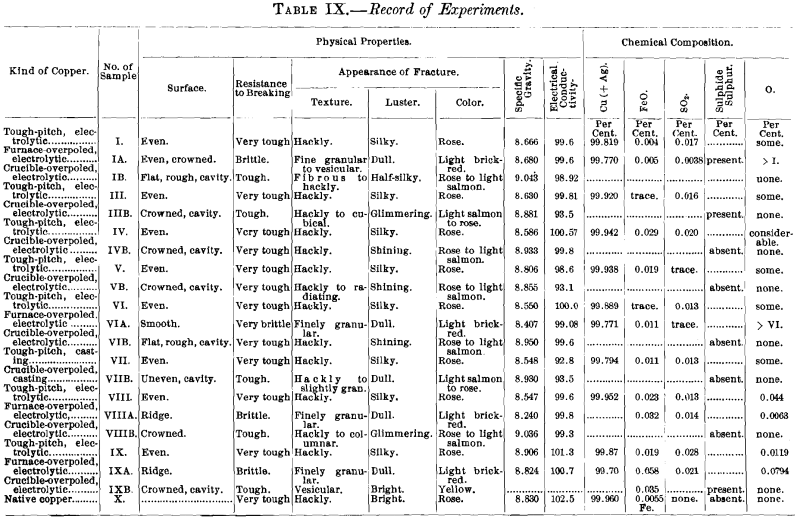
slightly burnt away. The entire absence of oxygen from the overpoled copper shows that the charcoal cover added had reduced any surface-oxidation of the charge that might have taken place. In melting down the first sample of tough-pitch copper, charcoal was charged with the copper. It was found, however, that some of the finer particles did not rise to the surface and made the copper cylinder rough and pitted. The main results are given in Table IX.; additional details of the tests are recorded in Table X.
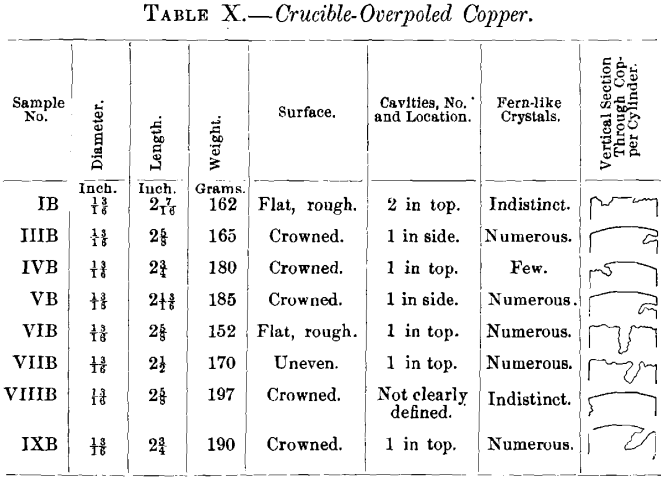
Table IX. shows that some samples of crucible-overpoled copper contain sulphide-sulphur. Its presence was determined by etching a polished sample with hydrofluoric acid. Under the microscope both cuprous oxide and cuprous sulphide show a bluish color, but, as first shown by Heyn, etching with hydrofluoric acid colors cuprous oxide black and leaves cuprous sulphide unchanged, thus making it easy to distinguish them from one another. Fig. 29 represents crucible-overpoled copper free from oxygen, with black spots of cuprous sulphide. In the experiments, Baker and Adamson’s c.p. hydrofluoric acid was used; 5 seconds treatment was sufficient to change cuprous oxide from blue to black. Attention may be called to
(a) Between samples 4 and 5, charcoal raked off, air admitted, fresh coal added to fire.
(b) Between samples 5 and 7, charcoal raked off, fresh fire made on grate.
(c) Metal too cold, adheres to poles; poles removed, fresh fire made, and poling started again.
the fact that Hampe had ascertained long ago by analytical methods that cuprous oxide, cuprous sulphide and sulphur di-oxide could be present together in tough-pitch copper.
The terms used in Table IX. to denote resistance to breaking require to be more closely specified. In breaking with a hammer a nicked sample clamped in a vise, the sample was termed very brittle when one blow was sufficient to break it, brittle when three or four blows were required, tough with more than four blows striking on one side, very tough with more than four blows striking on both sides.
The formation of a cavity when copper free from cuprous oxide is fused and cooled under reducing conditions, is a phenomenon to be expected, as copper shrinks upon cooling. Casting a bar of copper 3 by 3 by 30 in. on end with exclusion of air, J. B. Cooper obtained a pipe 5 in. deep, as shown in Fig. 23. When air came in contact with the surface of such a bar, when partly solidified, the surface rose immediately and finally crowned. The cross-section of such a bar, Fig. 24, 7 in. beneath the top, showed a core about 1 in. in diameter, which was crystalline and porous, while the balance was solid, resembling native copper.
A similar experience is that of Percy: Electrolytic copper melted under charcoal in a crucible and left to solidify therein showed no rise, but a depression. Similar copper melted under charcoal and poured, without taking any precautions to exclude air, gave a crowned surface.
While the samples used in the present experiments, weighing 162 to 197 g., did contain some cuprous oxide, and while its percentage may have been slightly increased during the first stage of fusion before the charcoal cover had been given, the microscopic examination of the specimens, which remained fused for 15 minutes under a charcoal cover and cooled in the crucible under it, showed that no cuprous oxide was present, and that therefore the reduction by charcoal had been complete. This is further brought out by the decrease in electric conductivity (see Table IX.) of all the high-grade samples of tough-pitch copper by crucible-overpoling, which must have reduced any oxide impurities present to the metallic state. A cavity was to be expected in the metal free from oxide, but the surfaces of all but one specimen (see Table X.) are also crowned. Crowning is due to the evolution of gases. That part of the gases held in solution by the tough-pitch copper have been completely eliminated in crucible-overpoling with the reduction of cuprous oxide and have not left the crucible-overpoled copper porous, is seen (Table IX.) by the rise in specific gravity of all the specimens from tough-pitch to crucible-overpoled copper. The samples of crucible-overpoled electrolytic copper then
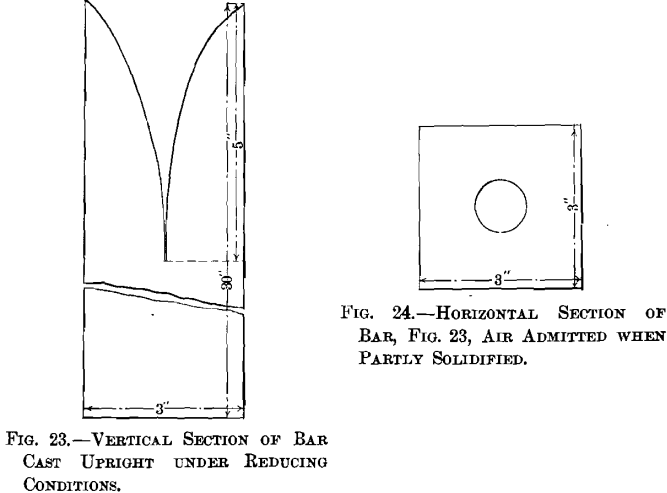
present the combination of a cavity due to cooling and a crown due to the evolution of gas.
Crucible-overpoled copper and native copper have only this in common, that they are both free from oxygen.
(c) Furnace-Overpoling.—When tough-pitch copper is cast from the reverberatory furnace into a mold, it gives an ingot, bar or cake with a level surface; when its surface shows a slight crowning, a ridge, or throws a worm (spews), it is sure to be overpoled. As shown above, the rising of the surface is due to the giving off of gas. Hampe found that copper had the property of absorbing sulphur dioxide, hydrogen and carbon monoxide, which rendered the metal porous. By heating copper charged with soluble gas in a current of carbon dioxide, which is insoluble, he expelled the dissolved gas and obtained a dense metal with a correspondingly higher specific gravity. Thus he raised the specific gravity of copper from Mansfeld, containing 0.075 per cent, of oxygen, from 8.525 to 8.906 by fusing in a current of carbon dioxide, the chemical composition of the metal remaining unaffected.
Caron had proved before Hampe that fused copper had the property of absorbing hydrogen and carbon monoxide.
Stein recovered from porous copper, by gently heating in vacuo, first hydrogen, then carbon monoxide. Heyn found that copper heated in a current of hydrogen to 600° C., became brittle and showed a decrease in specific gravity. Stahl examined three samples of slightly overpoled copper free from sulphur, taken from reverberatory-furnace charges just before casting. With a copper-content of 99.924, 99.893 and 99.899 per cent., the specific gravity was, 8.342, 8.466 and 8.266, while the specific gravity of the tough-pitch copper of the works averaged over 8.900.
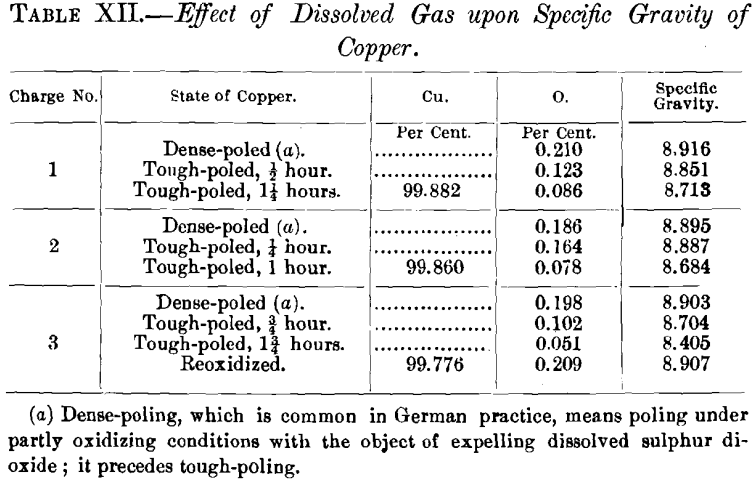
Perhaps the most striking examples of gas-absorption by copper while being poled are those shown in Table XII., given by Stahl, which show a decrease of oxygen with a decrease of specific gravity, while just the reverse would have taken place had it not been for the gas-absorption.
In poling, the charring of the wood sets free water-vapor, which stirs the copper, and carbon monoxide, hydrogen and hydrocarbons, which become more or less disseminated through it. As long as the copper is heavily charged with cuprous oxide, carbon monoxide and hydrogen cannot be retained by the copper, as they are oxidized to carbon dioxide and water-vapor, which are insoluble in copper; hydrocarbons are decom-
posed, the hydrogen is first oxidized and then the carbon. As the percentage of cuprous oxide decreases in poling, the oxidation of the gases diminishes, the absorption of carbon monoxide and hydrogen increases, and the finely-divided carbon from the decomposed hydrocarbons rises unoxidized to the surface of the metal-bath. The absorbing power of copper for gas increases with the temperature and the purity of the copper. According to Stahl, the gas-absorption becomes evident before the oxygen of the copper has been reduced to 0.07 per cent.; in one instance he noticed it when the copper still contained 0.160 per cent, of oxygen. Hampe found that the presence of the usual small amounts of impurity in copper did not affect the solubility of hydrogen, that the carbon monoxide was less soluble than hydrogen, and that cuprous oxide had no influence on the solubility of sulphur dioxide. Stahl’s experiments proved that lead, arsenic and phosphorus in amounts larger than common in refined copper decreased its dissolving power for gas; thus the addition of about 0.25 per cent, of lead or 0.4 + per cent, of arsenic or 0.024 per cent, of phosphorus toughened porous copper sufficiently to permit its being hammered, rolled or drawn.
The next question to be considered is, how does furnace-overpoled electrolytic copper differ chemically from tough-pitch electrolytic copper.
As to the oxygen-content, the data in Table IX. show that furnace-overpoled copper may have a higher or a lower percentage of oxygen than the corresponding tough-pitch copper. Thus, examples IA (Fig. 26), VIA and IXA (Fig. 28) contain more, and sample VIIIA less oxygen than the corresponding samples of tough-pitch copper I. (Fig. 25), VI., IX. (Fig. 27) and VIII. In Fig. 21, representing graphically the changes of sample No. VIII. (Table IX.), the tough-pitch copper, No. 18, was furnace-overpoled and three bars were cast at short intervals, giving specimens marked Nos. 19, 20 and 21, all of which contain less oxygen than No. 18. Crucible-overpoling Nos. 18 and 21 eliminated all the oxygen, as seen by Nos. 18a and 21a. In Table XI., the two furnace-overpoled samples contain less oxygen than the corresponding tough-pitch copper. Thus, of the six samples of furnace-overpoled copper, three contain more and three less oxygen than the respective tough-pitch copper. Refiners hold that most furnace-overpoled copper represents a false-overpole—i. e., the oxygen-content has been raised. Nevertheless, it is not an uncommon practice to slightly rabble copper that shows signs of crowning or tendencies to spewing in order to correct the evil. The rabbling increases the oxygen-content of the copper and thus diminishes its dissolving power for gas This practice is in line with the facts recorded above, in regard to the solubility of gas in copper.
As to the sulphur-content, Table IX. gives three samples of furnace-overpoled copper with a lower (IA, VIA, IXA) and one with a higher (VIIIA) percentage of sulphur than the corresponding tough-pitch copper (I, VI, IX, VIII). In Table XI., the four analyses of furnace-overpoled copper give more sulphur than the two of tough-pitch copper.
In regard to iron, Table IX. shows that the four samples of furnace-overpoled copper contain more iron than the tough-pitch copper. Why this should be so is not clear.
The influence of temperature is not definitely settled by the evidence of Table XI. In the first test, the temperature of the metal bath rose during the 1 hr. 5 min. of overpoling from 1,170 to 1,190° C., the copper became overcharged with gases and showed a median line when cast in a bar; air was then admitted (which did not increase the percentage of cuprous oxide) and coal added to the fire, when after 15 minutes’ additional poling at a temperature of 1,115° C., a bar cast spewed upon solidifying. These facts seem to prove that a temperature of 1,115° C. is sufficiently high for copper overpoled for 1 hr. 20 min. to hold enough excess-gas to make it spew upon solidifying when cast in the form of a bar.
In the second test, the copper, 1 hr. 51 min. after the tough-pitch stage, showed a ridge and then required only 5 minutes’ poling at a temperature 20° C. higher than before to throw a worm.
The two periods of overpoling, 1 hr. 20 min. and 1 hr. 50 min., of course, are excessively long, as under normal conditions from 5 to 10 minutes is sufficient to spoil the pitch.
