Alternative reagents for gold leaching include thiocyanate (SCN-) and thiourea (SC(NH2)2). They are considered less toxic and more environmentally acceptable when compared to cyanide. One of the important features of thiocyanate and thiourea dissolution of gold is that the leaching can be performed in acidic media, enabling the use of soluble oxidizing agents, such as ferric salts.
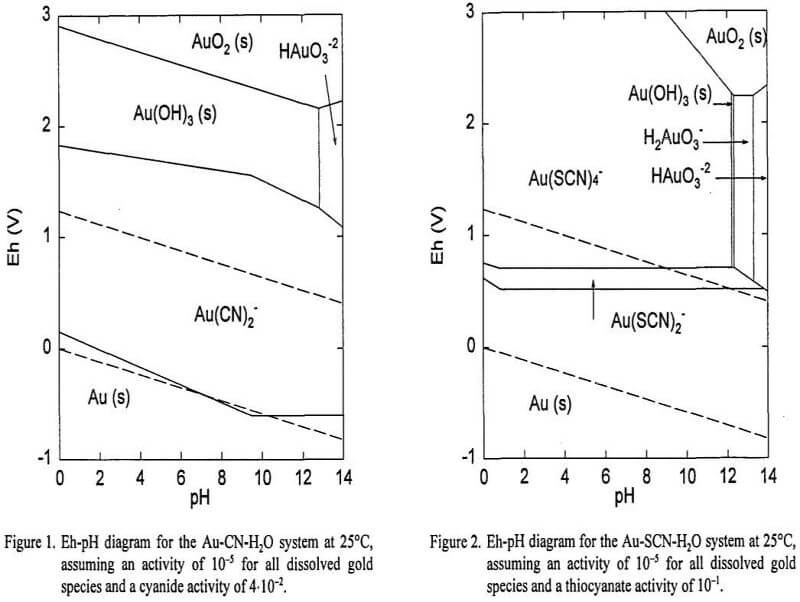
Gold leaching requires the oxidation of the metal in the presence of a ligand that can complex gold ions and stabilize them in solution. The complexing agents are necessary because the Au+ and Au+3 ions have very high standard reduction potentials, and these ions are not stable in simple aqueous solution. Certain ligands such as halides (Cl-, Br-, I-), pseudohalides (SCN-, CN-), and carbamides (CS(NH2)2) can reduce the stability of metallic gold by the formation of stable complexes that are soluble in water. In their presence, gold can be dissolved under modest oxidizing conditions within the water stability region. Gold forms aurous complexes with a coordination number of 2, and auric complexes with a coordination number of 4. Table 1 includes the standard reduction potentials, E°, for selected half-cell reactions. The stability constants, β, for the principal gold complexes in aqueous solutions are given in Table II.
Thiourea Leaching of Gold
The use of thiourea for gold leaching has been proposed as a process alternative due to the supposed lower toxicity, faster leaching kinetics and the alleged less interference from base metals as compared to cyanide (Sparrow and Woodcock, 1995). The overall reaction that describes gold dissolution in thiourea and ferric ion solutions is:
Au + 2CS(NH2)2 + Fe+3 ⇔ Au[CS(NH2)2]2+ + Fe+2……………………………………..[3]
In practice, thiourea leaching of gold is performed at thiourea concentrations of 0.13 M (10 g/L), ferric ion concentrations of 0-9·10 -2 M (0-5 g/L), pH values of 1-2, and potentials between 400 and 450 mV (SHE). Higher potentials during leaching produce an oxidative degradation of thiourea into formamidine disulfide (NH2(NH)CSSC(NH)NH2), which can decompose into thiourea (CS(NH2)2), cyanamide (NH2CN) and elemental sulfur (S°). By acid hydrolysis, thiourea can decompose into urea (NH2CONH2) and hydrogen sulfide (H2S). Elemental sulfur and hydrogen sulfide are undesired species during gold leaching. It is believed that both species cause a decrease in the leaching rate due to surface passivation, and H2S may cause reprecipitation of the gold (Sparrow and Woodcock, 1995). Figure 3 shows an Eh-pH diagram for the Au-thiourea-H2O system at 25°C, assuming an activity of 10 -5 for all dissolved gold species and a thiourea and formamidine disulfide activity of 1.5-10″1 (Lacoste-Bouchet et al., 1998).
NON Cyanide Gold Leaching Tests
The leaching experiments were performed with ore that was ground and dried to yield a size distribution with a d80 of 74 µm. Each leaching test was started using 250 g of ore plus 750 cm³ of leaching solution (25% solids by weight) in a 1-liter baffled glass vessel open to the air and at ambient temperature (23 ± 2°C). The pulp was agitated at 400 rpm with a stainless steel stirrer driven by an overhead motor.
In some experiments it was desired to chemically pretreat the ore. The purpose of the pretreatment was to dissolve the metallic sulfides and liberate the gold associated with these minerals. The ground ore was oxidized with concentrated nitric acid (69.5% w/w) at 60-80°C in a reactor stirred at 200 rpm to keep the solids in suspension. After 6 hours of treatment, almost all the metallic sulfides were dissolved with only the silicates and gold remaining. This oxidized ore was washed thoroughly to eliminate any residual acid and then dried. As a result of the chemical pretreatment, the ore weight decreased in about 22.5% The chemical pretreatment of 250 g of ore produced approximately 190 g of oxidized material, that was added to 750 cm³ of leaching solution (20% solids by weight).
Thiocyanate Leaching
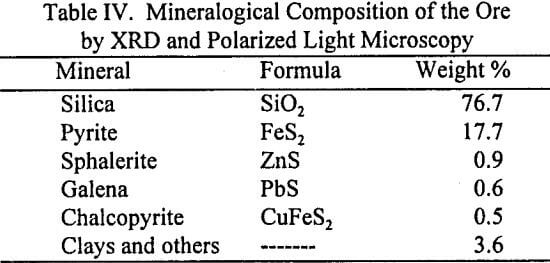
The variables for this study were the concentration of the lixiviant, added as sodium thiocyanate (NaSCN), the concentration of the oxidant, added as ferric sulfate nonahydrate (Fe2(SO4)3-9H2O), and ore chemical pretreatment, as shown in Table V. All experiments were carried out at pH 2.
Thiocyanate Leaching without Chemical Pretreatment of the Ore: Figure 5 illustrates the effect of [Fe+3]0 and [SCN-]0 on the overall gold dissolution from the ore without chemical pretreatment. The results show that for the range of variables studied, gold dissolution was dependent on both [SCN-]0 and [Fe+3]0. For both the low (0.04 M) and the high (0.08 M) values of [Fe+3]0, higher gold dissolution was achieved at the high value of [SCN-]0 (0.10 M). An overall higher gold dissolution was achieved with the higher [Fe+3]0 for both of the initial thiocyanate concentrations. In all cases the reagents were significantly in excess of that required for gold dissolution, and thus the extent of reaction is not limited by reactant depletion, as will be demonstrated later.
Chemical analysis of the solutions taken during the leaching experiments showed that there was an increase of total iron in solution (∆[Fe]) of about 10 to 13% after 24 hours of leaching. This means that the acidic thiocyanate-ferric sulfate system not only leached gold from the ore, but also dissolved iron from the pyrite. This is not surprising, as it is known that in acidic media, ferric iron leaches sulfides to produce elemental sulfur under certain circumstances:
MS + 2Fe+3 = 2Fe+2 + M+2 + S°………………………………………..[4]
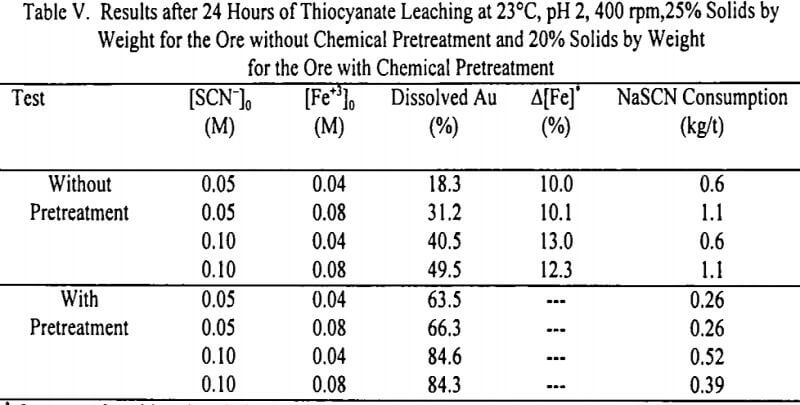
Thiocyanate Leaching with Chemical Pretreatment of the Ore: Figure 6 illustrates the effect of [Fe+3]0 and [SCN-]0 on the overall gold dissolution from the ore with chemical pretreatment. It shows that for the range of variables studied, gold dissolution was dependent on [SCN-]0 and less dependent on [Fe+3]0. For both the low (0.04 M) and the higher (0.08 M) values of [Fe+3]0, higher gold dissolution was achieved at the high value of [SCN-]0 (0.10 M). The initial ferric ion concentration was found to have little effect on the gold dissolution.
For all the combinations of [SCN-]0 and [Fe+3]0 studied, the percentage of overall gold dissolution obtained from the ore with pretreatment was higher than for the ore without chemical pretreatment. For the ore with chemical pretreatment, a maximum of 84.6% of the gold present in the ore can be leached in 24 hours, compared to a maximum of 49.5% for the ore without pretreatment. The dissolution rate was faster during the first 2 hours of leaching for the ore without pretreatment than for the ore with chemical pretreatment. During the next hour, the dissolution rate decreased for the ore without chemical pretreatment until it reached an equilibrium at 4 hours of leaching for [SCN-]0 of 0.05 M and at 8 hours of leaching for [SCN-]0 of 0.10 M. In the case of the ore with chemical pretreatment, the gold dissolution rate tended to be linear during the first 12 hours for [SCN‘-0 of 0.05 M and during the first 4 hours for [SCN-]0 of 0.10 M.
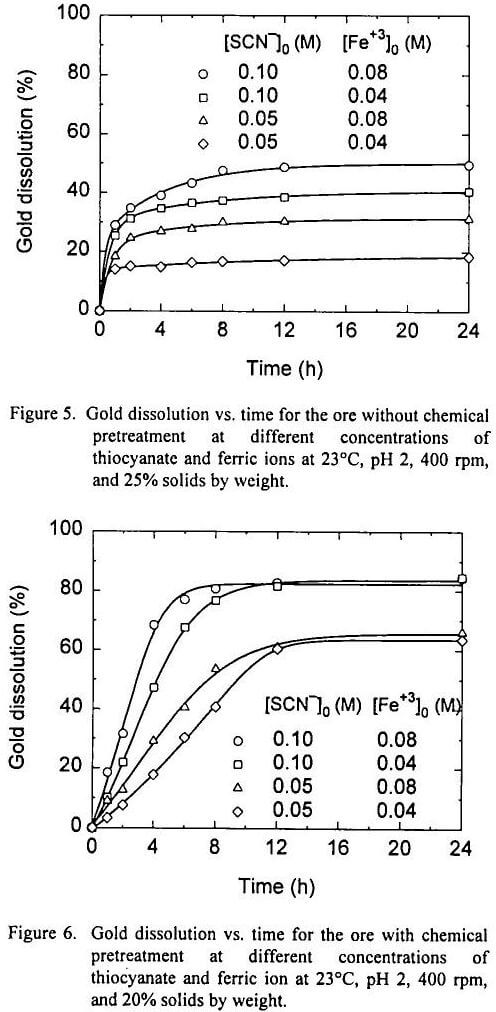
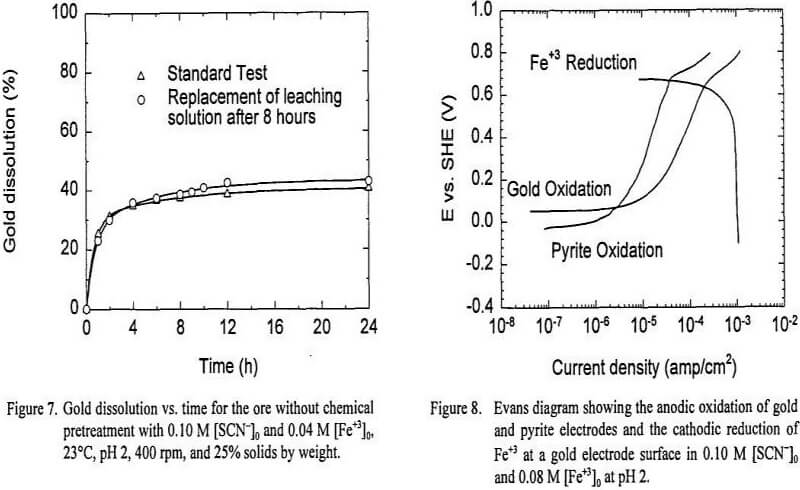
Recall that the total weight percent of dissolved gold with 0.10 M [SCN-]0 and 0.08 M [Fe+3]0 from the ore without chemical pretreatment is similar to the amount of free gold present in the ore (49.5% and 38.8% respectively). When the pyrite and other sulfides were dissolved from the ore, gold dissolution increased up to 84.3%. The results obtained from the first two experiments suggest that the thiocyanate-ferric sulfate system was able to dissolve free gold. Also, it is evident that the leaching solution is able to dissolve gold when free metallic gold is added to the system, and that the gold locked in pyrite was protected somehow from dissolution; although it is not likely that this was to galvanic protection, based on the electrochemical experiments.
Thiourea Leaching
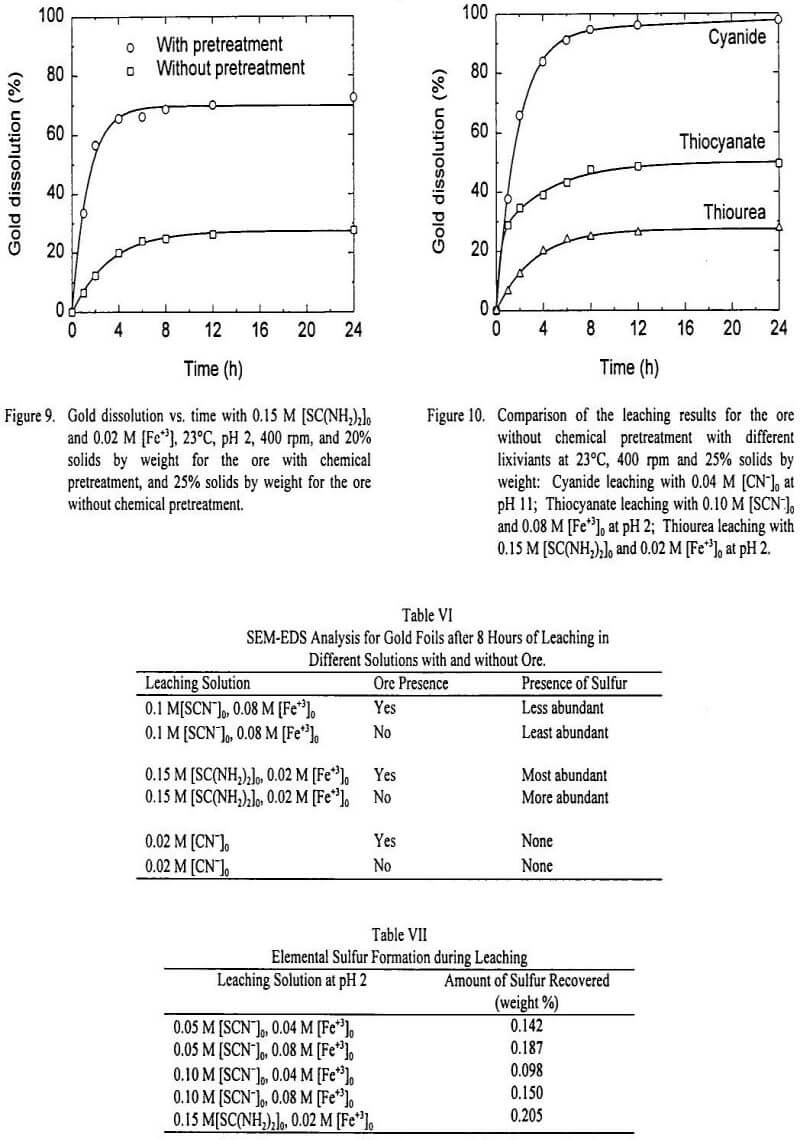
In order to compare gold dissolution from the ore with and without pretreatment for another lixiviant, the system of thiourea-ferric sulfate was chosen. Figure 9 shows the results for gold leaching at [SC(NH2)2]0 of 0.15 M (11.4 g/L) and [Fe+3]0 of 0.02 M (1.12 g/L) and pH 2. As with the thiocyanate lixiviant, gold dissolution of the ore without chemical pretreatment was very low, compared to the ore with pretreatment. Without chemical pretreatment, 27.6% of the gold was dissolved, while with chemical pretreatment, the overall dissolution was 72.5%. Thiourea consumption was high in both cases, of 38.3 kg/ton and 36.8 kg/ton for the ore without and with chemical pretreatment, respectively.
Comparison of Cyanide Thiocyanate and Thiourea Systems
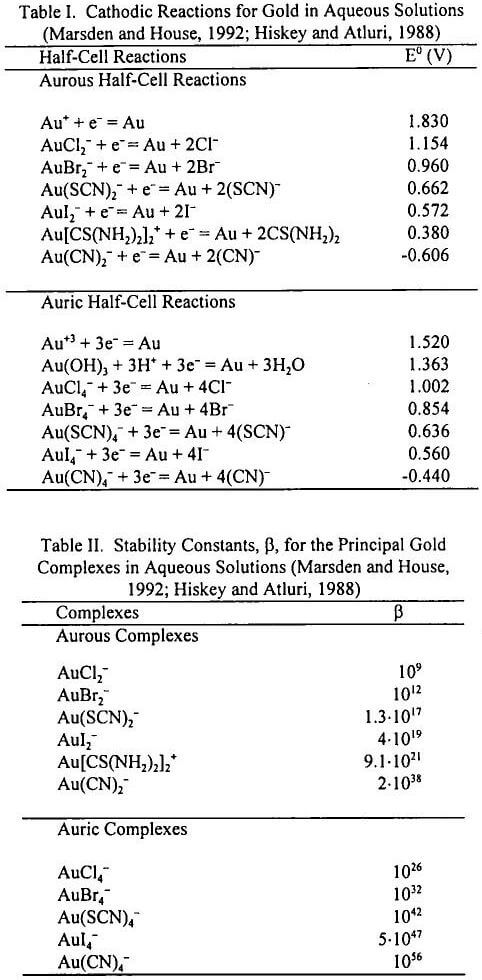
Figure 10 compares the results obtained after 24 hours of leaching of the ore without pretreatment by the three systems. Cyanide leaching with 0.04 M [CN-]0 at pH 11 resulted in a better overall gold dissolution (97.6%) than thiocyanate leaching with 0.10 M [SCN-]0 and 0.08 M [Fe+3]0 at pH 2 (49.5% gold dissolution) or thiourea leaching with 0.15 M [C(NH2)2]0 and 0.02 M [Fe+3] at pH 2 (27.6% gold dissolution).