Table of Contents
This gas can be prepared from ammonium nitrate, which can be made by neutralising dilute nitric acid with ammonium hydrate. Take some of the nitric acid left over from the last experiments, dilute it with a little water, put it in a porcelain basin, and add ammonium hydrate to it, keeping it stirred with a glass rod until a drop taken on the rod ceases to redden blue litmus paper. Set the basin over a low bunsen flame and evaporate down slowly, adding from time to time a few drops of ammonium hydrate to keep the solution alkaline, until the liquid becomes slightly viscid and shows no signs of ebullition. Set the basin aside and allow to cool.
NH4HO + HNO3 = NH4NO3 + H2O
To prepare the gas for experiments, fix up your apparatus in a way similar to that for the preparation of oxygen. Take about 10 grams of ammonium nitrate and place carefully in the bottom of your flask. Fill your pneumatic trough with warm water instead of cold, as N2O is soluble in cold water, and set up four gas jars in the trough as before.
Heat the flask gently until the salt fuses, then gradually increase the heat until the fused ammonium nitrate begins to decompose with effervescence and bubbles of gas pass up rapidly through the water in the trough. Allow time for the air in the flask to be displaced, then begin to collect the gas. In heating the flask do not allow the temperature to get too high; if many white fumes arise in the flask, moderate the heat, and when you have collected sufficient gas, remove the cork and delivery tube from the flask to prevent the water rushing up the tube and cracking your flask.
NH4NO3(heated) = N2O + 2H2O
Nitrogen Monoxide Laboratory Experiment I
Take one of the jars of gas from the trough and plunge a lighted taper into it; the flame burns brighter than in air, but the gas itself does not take fire.
Nitrous Oxide Laboratory Experiment II
Place a piece of amorphous phosphorus in a deflagrating spoon, ignite it, and plunge it into another jar of the gas; the phosphorus burns almost as brilliantly as in oxygen.
5N2O + P2 = P2O5 + 5N2
After the fumes of P2O5 have subsided you can test the remaining gas in the jar for nitrogen by plunging a lighted taper into it.
Laboratory Experiment III
Place a small piece of sulphur on the deflagrating spoon, just light it and plunge it into another jar of the gas; the flame is extinguished, the temperature of the burning sulphur not being high enough to break up the gas. Heat up the sulphur now considerably above its melting point and plunge it into the same jar of gas; it burns brilliantly.
2N2O + S= SO2 + 2N2
Laboratory Experiment IV
Pour a little cold water into the remaining jar of the gas, close the mouth with the hand and shake briskly; invert the jar, still keeping the hand over the mouth, and bring it beneath some cold water in another trough; withdraw the hand and you will see the water rise in the jar; repeat the shaking, and again bring the jar under cold water, and you will see the water rise still more; this shows the solubility of nitrogen monoxide in cold water.
Nitrogen Dioxide – Nitric Oxide Gas
Weigh out about 30 grams of copper shavings, put them into a flask fitted with a thistle funnel and delivery tube, and fix up your apparatus in a similar way to that for the preparation of hydrogen. Mix abuut 60 c.c. of strong nitric acid with about the same quantity of water and pour carefully down the funnel; red vapours will almost immediately till the flask, but will disappear after the air has been displaced in the flask.
3Cu + 8HNO3 = 3Cu(NO3)2 + 4H2O + 2NO
Collect four jars of the gas.
Nitrogen Dioxide Laboratory Experiment I
Plunge a lighted taper into a jar of the gas; the taper goes out and the gas does not take fire, but reddish-brown fumes appear in the jar.
Nitric Oxide Laboratory Experiment II
Place a small piece of amorphous phosphorus in the deflagrating spoon, light it and plunge it immediately into a jar of the gas; the flame is extinguished. Again light the phosphorus by holding it for a few moments in the bunsen flame, and when it is burning briskly plunge it into the jar ; the phosphorus burns with increased brilliancy.
5NO + P2 = P2O5 + 5N
Laboratory Experiment III
Plunge a piece of brightly burning sulphur into another jar of the gas ; it is extinguished.
Nitric oxide takes a higher temperature to break it up than nitrous oxide.
Laboratory Experiment IV
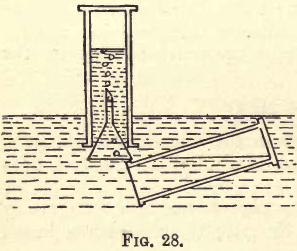
Prepare a jar full of oxygen; decant about one-fourth of the nitric oxide in the remaining jar into another gas jar by means of the trough, and very slowly pass up into this a little oxygen (fig. 28). As the oxygen comes in contact with the nitric oxide, reddish-brown fumes are produced. After adding a small quantity of oxygen, shake the jar containing the mixed gases, keeping its mouth under water to prevent air from getting in; the water will rise, showing the gas is soluble. Continue the addition of oxygen, shaking the jar at intervals ; after a time the jar will become entirely
filled with water.
2NO + O2=2NO2
The NO2 gas is readily soluble in water. Filter the liquid remaining in the flask and transfer to a porcelain basin, then evaporate it down to about one-third of its bulk and allow the crystals of copper nitrate to crystallise out. These crystals will be of a bluish colour.