Table of Contents
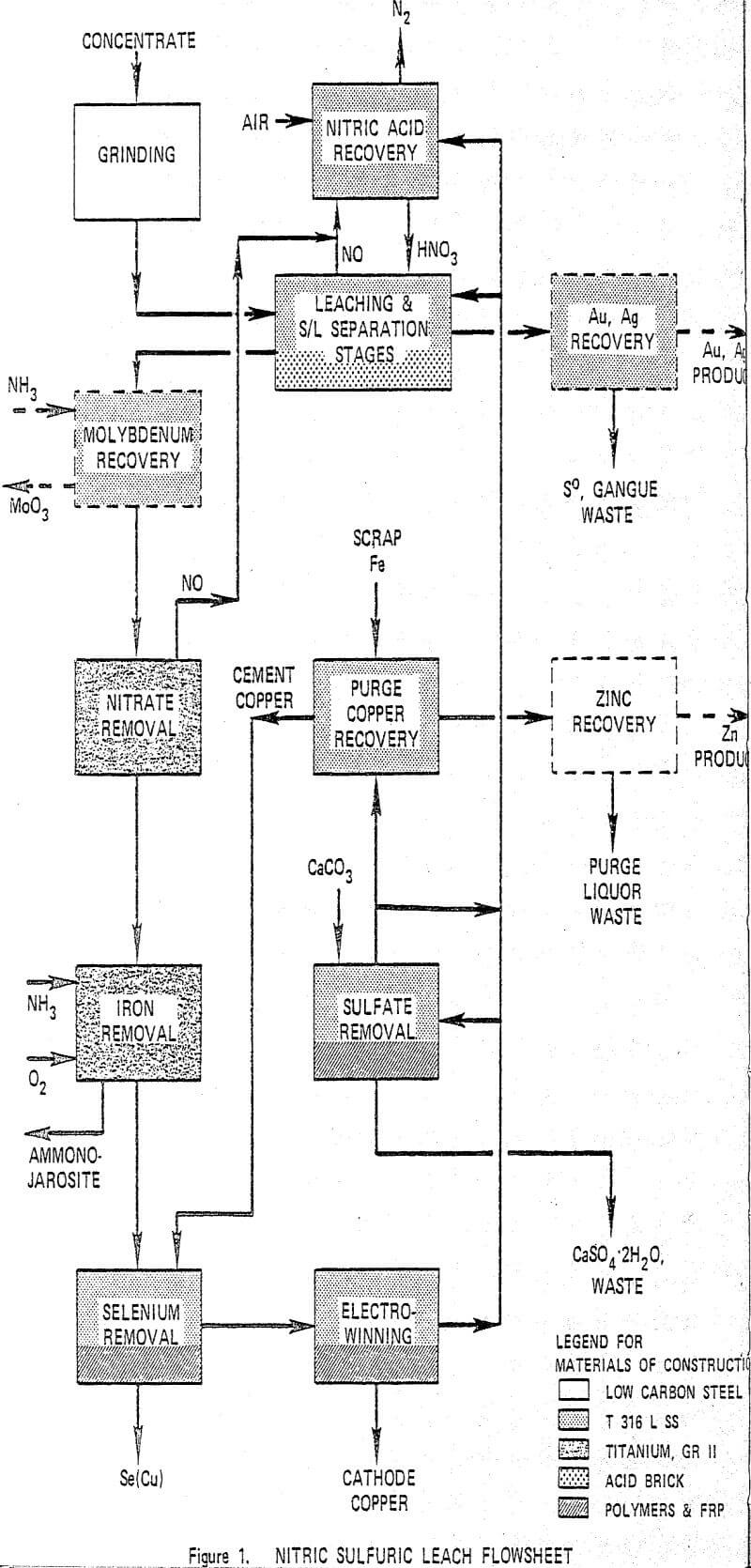
Copper is leached in a staged reactor system utilizing nitric and sulfuric acids at 105°C. Iron is removed from the pregnant liquor as a jarosite and cathode copper is electrowon directly from the purified pregnant liquor. The spent electrolyte is recycled. Nitrogen oxides evolved from the leach are reconverted to nitric acid and are also recycled. Sulfur is rejected as S°, jarosite and gypsum. The process is highly versatile and can recover precious metals, molybdenum and zinc, when these elements are present.
Nitric Acid Processes
The use of nitric acid and mixed nitric-sulfuric acids as a hydrometallurgical process leach medium has been known since the early 1900’s. Recent work, based on the nitric-sulfuric leach systems has been described by Prater et al and Bjorling et al for copper concentrates and by Ouellet et al for nickel concentrate. In these processes, oxidant is supplied by the breakdown of nitrate ion in acid solution.
1½ MeS + 4 H+ + NO3 → 1½Me++ + S° + 2H2O + NO↑
S° + 2 NO3- → SO4= + 2NO↑, ΔH = -70 kcal/gmol
2NO + O2 → 2NO2, ΔH = -27.6 kcal/gmol
H2O + 3NO2 → 2HNO3 + NO, ΔH = -32.9 kcal/gmol
The regenerated nitric acid is returned to leaching along with sulfuric acid regenerated during the winning of the copper.
Process Description
Feed concentrate is ground to an optimum particle size for the leaching and solid/liquid separation requirements of the leach step which follows. In the leach step, essentially all of the metal sulfides in the concentrate are dissolved while reducing the concentration of nitrate and free acidity in the pregnant liquor to low levels. Nitric oxide from the leach reactors is oxidized in the nitric acid recovery step and absorbed to regenerate HNO3. If molybdenum is present, it is completely solubilized during the leaching and is removed from the pregnant liquor in the next step by liquid ion exchange. The pregnant liquor is then fed to the nitrate removal autoclave where residual nitrate is reduced to NO, and Fe+² is simultaneously oxidized to Fe+³. Filtrate from the iron removal step is contacted with recycle cement copper to precipitate selenium and the purified liquor is fed to electrowinning where cathode grade copper is recovered.
Laboratory Work
A combined technical and economic analysis of the process based on the laboratory results followed. It showed that the process had about 10% lower capital requirements and about the same operating costs as compared to continuous smelting processes and 10 to 20% lower capital and operating costs as compared to developed hydrometallurgical processes.
A second phase of laboratory work was then initiated based on an integrated bench scale operation of the process using Kennecott concentrate. It employed every step of the process shown in Figure 1 except for nitric acid recovery and for purge copper and zinc recovery. Concentrate was ground in a batch mill. Solutions were made up to precalculated concentrations of the major constituents and operations were continuous for each process step.

Process Parameters
Grinding of ore gives substantially increased initial leach rates which reduces reactor residence time and improves control of the first stage leach reactor. Overgrinding can create solid-liquid separation problems. A limit of 95% minus 325 mesh was found to be an acceptable compromise of leach rates and control characteristics with filtration and settling unit areas. The leach step is designed to solubilize 99% or more of the copper contained in the concentrate. The leach is carried out in a three-stage counter current arrangement with solid-liquid separation between each stage.

The initial laboratory work which led to selection of leach conditions demonstrated that leach rates increase with concentration of NO3-, H+ and partial pressure of NO. The reaction mechanism is complex and for the step 1 reactor, careful control of concentrate feed is required to maximize the utilization NO3- and H+. Reaction rates also increase with temperature but have a practical upper limit imposed by the 120°C melting point of sulfur. However, acceptably fast reaction rates were found for the 90 to 105°C range which avoided pressure reactors and allowed the use of brick lined vessels with stainless steel trim.
Removal of selenium is required to meet cathode copper quality requirements. Selenium is partly rejected in the leach tails and partly precipitated with jarosite in the nitrate and iron removal steps. Approximately 40% of the selenium contained in the concentrate remains in solution after iron removal and is precipitated by reaction with recycle cement copper. The principal reactions are:
2Fe+³ Cu° → 2Fe+² + Cu+²
H2SeO3 + 4Cu° + 4H+ → Cu2SE + 2Cu+² + 3H2O
At a temperature of 70°C, reaction was found to be rapid requiring a holdup time of 10 minutes to complete the removal of the selenium. It is important that acid content be maintained above 30 g/l.
Conventional electrowinning at approximately 200 amps/m² of cathode is used for recovery of cathode grade copper. Copper content is decreased from a feed level of 80 g/l to an effluent concentration of 30 g/l. Critical impurities are removed from electrolyte prior to electrowinning. Cathode lead content is controlled by use of cobalt addition and by limiting current density to insure high quality deposits.
The majority of the sulfur converted to sulfate in the leach step is removed as jarosite in the iron removal step. The balance appears as excess sulfuric acid and is removed from the copper depleted electrolyte by reaction with limestone to precipitate gypsum. The reaction is carried out in a crystallization reactor using a holdup time of approximately 2 hours at a temperature of 70°C.
Energy Requirements
A review of the energy requirements (on a fuel basis) for hydro and pyrometallurgical processes shows that, in general, the hydrometallurgical processes require more energy than the pyrometallurgical processes. The nitric-sulfuric leach process requires about 2.5 times as much energy as flash smelting. A large portion of the additional energy is consumed in the copper electrowinning step. A typical flash smelter requires about 15,000 Btu/lb to process concentrate to cathode, whereas, the nitric-sulfuric leach process requires about 35,000 Btu/lb. Electrowinning power accounts for 45% of the total.
Nitric Sulfuric Leach Process Improvements
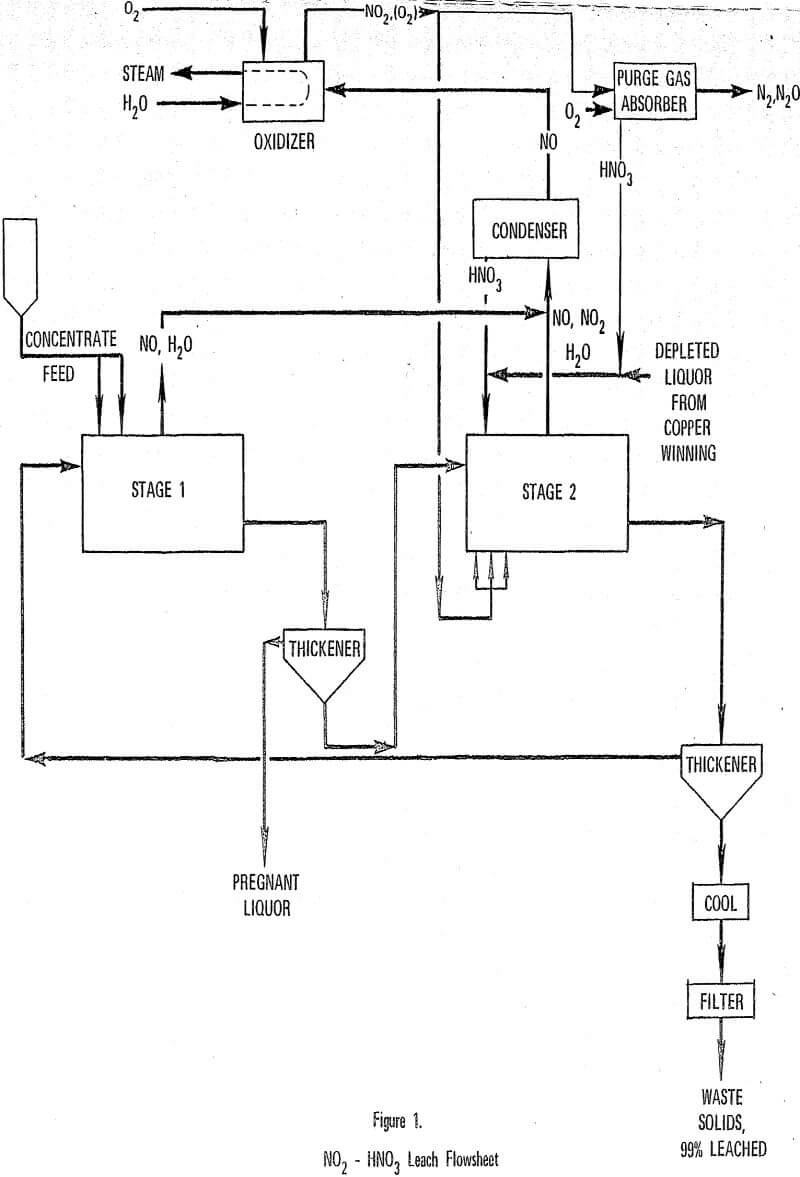
Further development of the Nitric-Sulfuric Leach (NSL) process has led to an improved design of the leach and nitric acid recovery steps. NO produced in the leach step is reacted with oxygen, regenerating NO2, which is sparged directly into the leach reactor. Leaching is simplified to two steps and the nitric acid plant is replaced with an oxidation recycle gas system. Laboratory work substantiated the concept and led to selection of operating conditions.
Background
In the search for a hydrometallurgical process to replace smelting for the production of copper, the nitric-sulfuric leach process described by Brennecke et al was developed as an alternative which meets all the requirements for processing most copper concentrates. The major technical advantages of the process are reviewed below.
- It utilizes a powerful and regenerable oxidant, nitric acid, to completely (99%) leach copper and other heavy metals from all sulfide concentrates.
- It takes advantage of sulfuric acid process characteristics by rejecting iron as jarosite and recovering cathode copper directly from purified pregnant liquor.
- By-product metals are recoverable.
- Process wastes are stable and should be compatible with disposal requirements.
Economic analysis showed that the process had comparable capital costs ($2400/AT) and lower operating costs (34¢/lb Cu) as compared to other hydrometallurgical processes. It compared favorably with flash smelting for plants smaller than about 50,000 TPY of copper; however, the costs were not sufficiently attractive for NSL to replace smelting unless some other imperative such as environmental constraints, low grade concentrates, ores not amenable to smelting, or unique by-product recovery capability would justify the risk of commercializing the new technology.
NO2 Leach Process
In the revised leach-NO recovery process, NO2 is sparged directly into the leach reactor where it contacts a slurry of mineral concentrate in mixed nitric-sulfuric acid leach liquor. The mineral is leached in accordance with the stoichiometry shown in reaction.
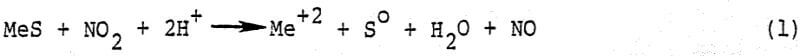
![]()
![]()
The leach is conducted in a 2-stage (as compared to the original 3-stage) countercurrent arrangement with solid-liquid separation following each leach reactor. As with the original NSL process, the purpose of the 1st stage leach is to react fresh concentrate with 2nd stage liquor to produce a pregnant liquor with less than 10 g/l each of NO3- and H2SO4.
Experimental Program
When NO2 is sparged into an aqueous liquor, it reversibly absorbs and reacts according to the following reactions.
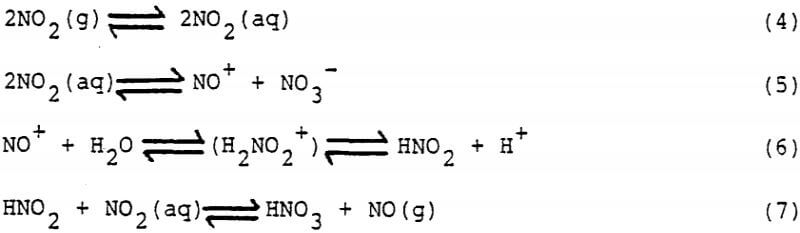
Leaching was conducted in a 1.52, resin kettle reactor generally using 600 ml of leach solution and 100 g of concentrate per experiment. Gas flows in and out were monitored using Matheson electronic mass flow meters. An automated gas chromatograph and a chemi-luminescent NOx analyzer were used to determine NO, NO2, O2 or Ar, N2, N2O and H2O in the offgas.
The results showed that absorption was not dependent on acid concentration over the range studied (0 to 74 gms HNO3/liter). Absorption rate of 3.0 x 10 -4 g/min/ml solution for run 9-1 is approximately half that required for a commercial reactor. In a second run, 9-2, 150% excess oxygen was added with the NO2 and quantitative absorption resulted. The data indicated that NO2 absorption would not be a problem either in the test work or in a commercial reactor where reactor depth is favorable to absorption efficiency.
NO2 Leaching
Concentrate leaching experiments were conducted in a manner analogous to the NO2 absorption tests except that NO2, and in some cases NO2+O2, was sparged into an agitated slurry rather than into clean solution.
When NO2+O2 was used as the oxidant, a modest increase in sulfide oxidation rates was observed. This most likely arises from an increase in the partial pressure of NO2 in the reactor. An increase in final [HNO3] is also observed, but most of the leach rate enhancement occurs while [NO3-] is quite low.
- Leaching at 90°C was more effective than leaching at 100° C.
- Increasing initial acidity from 20 to 100 g/l H2SO4 inhibits sulfide leaching to a small but significant extent.
- Under most conditions, the enhanced leaching rates in the presence of NO2 + O2 are not very sensitive to changes in the NO2/O2 ratio.
Electrowinning
In air agitation electrowinning, air induced convection of electrolyte increases the diffusion rate of dissolved copper to the cathode surface and allows use of higher current densities while producing physically sound deposits. The high rate of convection also sweeps impurities from the face of the cathode such that fine solids impurities do not become occluded in the deposit. The result is a physically sound cathode of high purity.
The Kennecott air agitation process utilizes close anode-cathode spacing (2.5 cm) both to increase convection per unit volume of air and to reduce cell voltage. Cathode blanks of stainless steel or titanium are sufficiently larger than the anode so as to eliminate edge deposits and the need for plastic edge strips on the blank.
Air agitation electrowinning was originally tested in a liberator cell at our Baltimore refinery alongside cathodes without air agitation.

