Table of Contents
The process parameters for effective utilization of nitric acid as an oxidant for copper-iron sulfides have been developed. Leaching variables found to be important were acid concentration, temperature, time, atmosphere, pressure, mineral type, particle size, and percent solids. Particular attention was devoted to analysis and control of fumes evolved during the oxidation.
Numerous references have cited the effectiveness of HNO3 in oxidizing copper sulfides. The gaseous reaction products reported were NO and NO2).
3CuS + 2HNO3 + 3H2SO4 → 3CuSO4 + 3S° + 2NO↑ + 4H2O
CuS + 2HNO3 + H2SO4 → CuSO4 + S° + 2NO2↑ + 2H2O
Experimental Technique
Most of the leaching experiments were performed in a one-liter glass reaction kettle equipped with condenser, cooling coil, agitator, pH probe, heating mantle, thermometer, acid burette, and sampling tube. The sulfide, Concentrate “A”, used for the experiments assayed 28 percent Cu, 25 percent Fe, 31 percent S, 0.29 percent Mo, 3.5 oz Ag/ton, and 0.4 oz Au/ton. The major mineral constituents were chalcopyrite (60 percent), bornite (10 percent), gangue (14 percent), and pyrite (10 percent). When pure mineral sulfides were employed, they were natural specimens ground and sized to meet experimental requirements.
Elemental sulfur was extracted from leach residues with carbon di-sulfide prior to flotation. The residue was then ground in the presence of sufficient lime to give a pH of 8. 0 to 8. 5 during the subsequent flotation treatment. Reagents employed during the 8-minute float were xanthate Z-6 collector (0. 2 lb/ton) and MIBC frother (0.3 lb/ton). The type and quantity of reagents used during flotation were not optimized.
The Leach Step
Four responses were used as criteria for optimization of the leaching step: (1) metal extraction, (2) elemental sulfur yield, (3) iron rejection, and (4) reagent consumption. Preliminary experiments showed that these responses were dependent upon the variables of acid concentration, temperature, time, percent solids, mineral type, particle size, atmosphere, and pressure; agitation rate was not a significant variable. For most of the experiments, mineral type, particle size, and pressure were held constant by leaching the sulfide concentrate defined above at atmospheric pressure with oxygen absent. Both full and fractional factorial designs were used to study the effects of HNO3 concentration (10 to 60 percent), H2SO4 concentration (0 to 2 M ), temperature (75° to 95°C), time (30 to 240 minutes), and percent solids (15 to 33 percent).
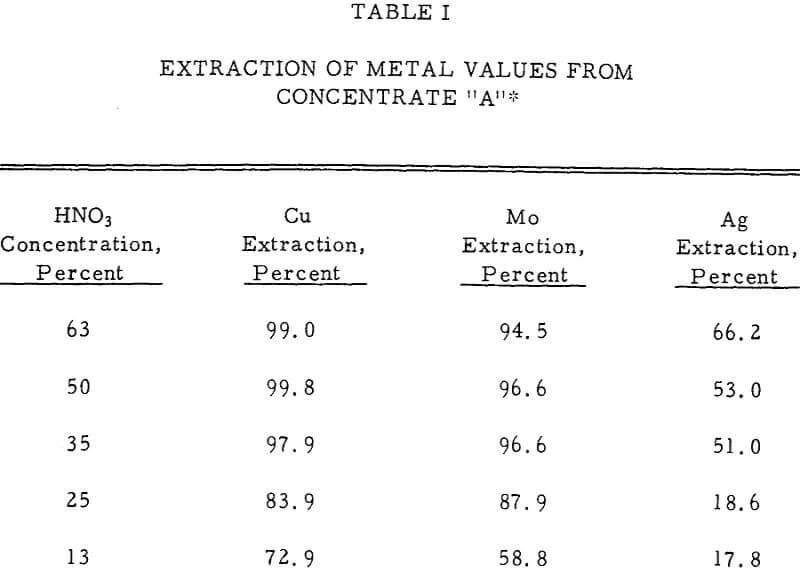
A primary goal of most metallurgical processes is high extraction of the metal values from the feed material. In the HNO3 leaching system, acid concentration, mineral type, particle size, temperature, and time were all important factors in achieving this goal.
A problem common to most processes recovering copper from sulfide ores is the rejection of iron. Previous investigators of the nitric acid system utilized iron hydroxide precipitation to accomplish this end; however, this precipitate is difficult to filter and causes sliming problems during flotation.
Pressure Leaching Attractive
Autoclaving is not necessary to successfully leach with HNO3. Pressure does, however, have a number of attractions:
- Increased pressure accelerates the regeneration of HNO3 within the leach tank.
- Evolution of NOx gases already under pressure eliminates the need for off-gas heating, compression, and cooling in preparation for absorption.
- The higher leach temperatures attainable under pressure maximize S° formation, favor hydrogen jarosite formation, and minimize residual nitrate concentration.
The chemistry learned at atmospheric pressure is presently being applied in a pressurized system.
Process Development
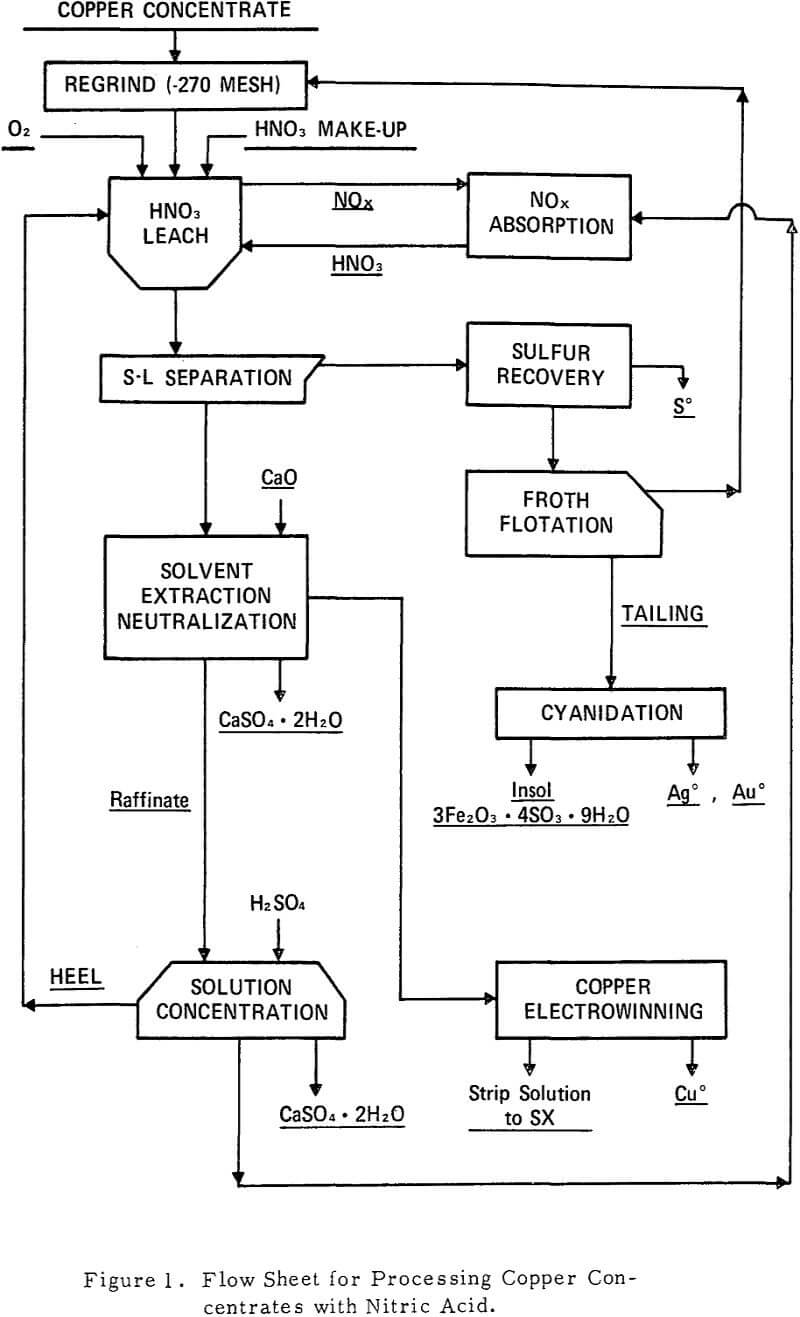
An ideal leach would extract all of the copper, precipitate all of the iron, convert all the sulfide to S°, and consume all of the nitrate added. Unfortunately, the chemistry of the HNO3 leach system does not appear to simultaneously offer these ideals. The conditions chosen which would most nearly meet these ends were the addition of oxygen and a deficiency of HNO3 to a slurry of copper concentrate at 90°C. Present in the slurry were fresh concentrate, recycled leach residue, and a solvent extraction raffinate. Over a 1.5-hour period, 1.6 pounds of HNO3 were added for each pound of copper to be oxidized. The continuous addition of HNO3 as a 50 percent solution avoids excessive acid concentration at any time during the leach. One-half hour after completing this addition, the slurry temperature was raised to the incipient boiling point and the oxidation allowed to continue for the next 2 hours.
The gas evolved from the leach as described under “Optimum Leach Conditions” is almost entirely NO and water vapor; the NO2 content averages 2 percent and the N2O 1 percent. The N02 content increases as injected oxygen reacts with the NO. Traces of N2 were detected, but these were probably contaminants from air. Hydrogen sulfide appears only when pyrrhotite is treated, and then only when copper is absent.
Six cycles according to the flow sheet were completed. In each cycle, 80 percent of the copper and iron fed to the leach was solubilized. Ninety percent of the solubilized iron subsequently precipitated into the leach residue as hydrogen jarosite. Sixty percent of the sulfide sulfur oxidized during the leach was converted to S°. Two-thirds of the remaining sulfur precipitated with the iron as hydrogen jarosite, and one-third was precipitated from the solvent extraction raffinate with lime. The HNO3 leach residue assayed 5.6 percent Cu, 25 percent Fe, and 14 percent S°.